N 1 2 3 8. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital.

How To Determine The Maximum Number Of Electrons Given A Set Of Quantum Numbers Youtube
You just pretend to and then in second-year you learn them.

How to find electrons given quantum numbers. 1 2 3 4. Quantum numbers are considered as the coordinates that will give an idea about the location of an electron in an atom. There are four different quantum numbers.
Here we want to find the number of electrons that an atom can have for some given quantum numbers. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12. Number of Electrons in Shells.
And in each one of these orbitals we can hold a maximum of two. Principal Quantum Number n has integral values 1 2 3. We use this formula to find m.
A really simple way to think about this is just knowing that the angular quantum number l specifies the subshells. Each electron in an atom is described by four different quantum numbers. Filling the electrons according to our rule we observe that the 21st electron occupies the 3d sub-shell.
4 Marks a 5. Consider Scandium Sc with its atomic number of 21. Is that the values of.
Electrons in the same atom that have the same principal quantum number are said to occupy an electron shell A term used to describe electrons with the same principal quantum number. If you add up all the electrons in the shell n5 you will get a total of 50 electrons. The first quantum number is the principle quantum number which is.
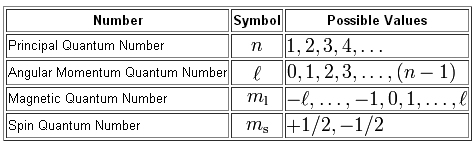
The Quantum Numbers can give both the quantity and location of electrons within a given atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Dont worry nobody understands these in first-year chemistry.
However as the previously filled 4th shell 4s has 2 electrons and is apparently the outermost shell the number of valence electrons is 2. Please include an explanation as well as any formulas rules. We know that for an atom with quantum numbers n and l the orbitals can take the values from -l to l so we will have 2l 1 orbitals.
The principal quantum number of electrons in the D-block is period minus 1. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that can occupy the orbital. The principal quantum number can be any nonzero positive integer.
L can take values from 0 to n. Only one electron in a given atom can have the set of quantum numbers given in the question. I If the element belongs to s-block its group number Number of valence electrons or outermost shell electrons.
According to the Pauli Exclusion Principle no two electrons can share the same combination of four quantum numbers. Give the maximum number of electrons in an atom that can have these quantum numbers. Half of these electrons are spin up ms12 and half these electrons are spin down ms-12 so the maximum number of electrons that can have the quantum numbers of n5 and ms12 will be 25 electrons.
Quantum Theory Electrons Concept 1. The rule for m. B n 1 0 m 0.
The principal quantum number of electrons in the F-block is period minus 2. Quantum numbers of an electron. Each electron in an atom has a unique set of quantum numbers.
The total number of electrons having magnetic quantum number m 0 is 14. Angular Momentum Quantum Number l associated with the number of. Quantum Numbers Maximum Number of Electrons.
If l 0 it is the s orbital which has 1 subshell and can therefore hold two electrons. For the quantum number. This number determines the size and energy of the orbital.
Now lets see how to get that answer. We will see that this atom can hold 14 electrons. Spans from negative to positive with 0 in between.
If only n is given you can just use the formula 2n2 to determine the number of electrons. Ii If the element belongs to p- block group number no. If l is given it can mean multiple things.
Of valence electrons 10 ie 10 np electrons ns electrons. Principal Quantum Number n. The first quantum number is n telling the shell in which the electron is present.
Quantum numbers are important because they can be used to. Values below how many possible values are there for the quantum number m. No two electrons in an atom can have the same set of quantum numbers.
The second quantum number is l telling the subshell in which electron is present. A n 3 2 m -1 ms 12.

Quantum Numbers The Easy Way Youtube

Quantum Numbers Video Quantum Physics Khan Academy

Orbitals Quantum Numbers Electron Configuration Multiple Choice Practice Problems Youtube

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
Quantum Numbers Introduction To Chemistry