Fluorine is the most electronegative component as a result of it has 5 electrons in it is 2P shell. A n 2 ℓ 0 m ℓ 1 m s -12.

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
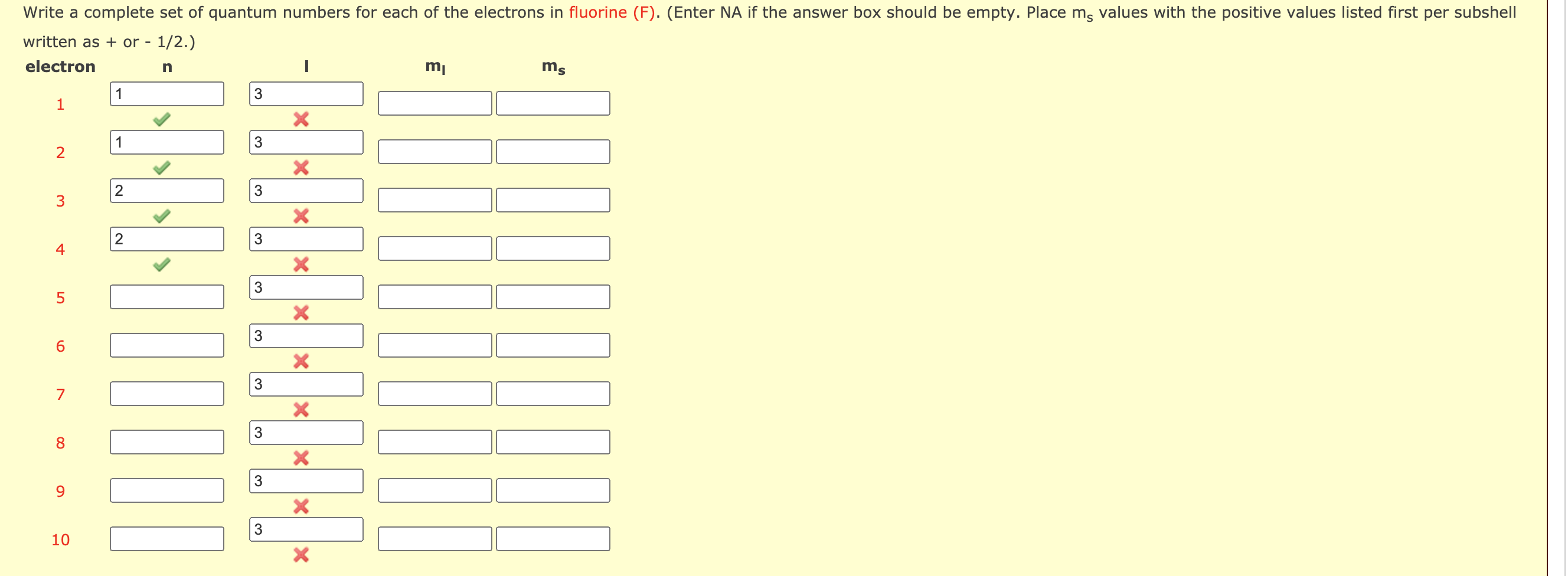
The values of the four quantum numbers for each of the nine electrons of fluorine are.

What is the set of quantum numbers of fluorine. N 1l0ml 0ms 12 n 1 l 0 m l 0 m s 1 2. Each quantum number is then assigned according to a set of rules each of which took years of study to finally determine. 1 H - Hydrogen.
C n 3 ℓ 1 m ℓ 1 m s 12. Fluorine can gain an electron to become fluoride F. An electron is described by four sets of quantum numbers.
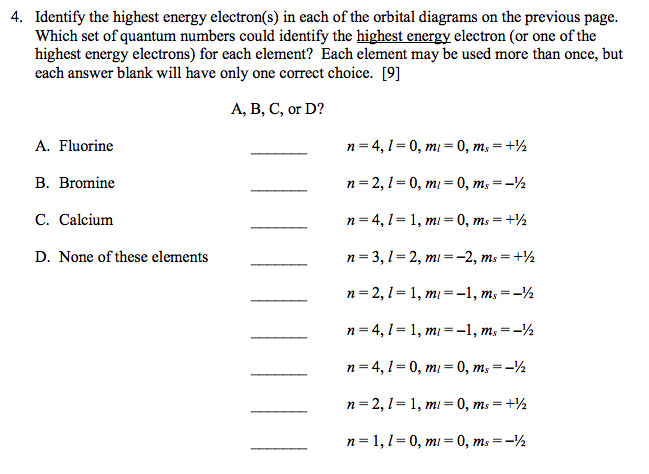
The second shell is the highest energy shell and the 2p 2 p is the. Click hereto get an answer to your question 1. A A B B C C D D E E Ans.
N 3 l 2 m l -1 m s 12 III. N 4 l 1 m l 1 m s 12 II. Hence the correct set of quantum numbers is.
You can thus say that the full quantum number set that describes the 9th electron in an atom of fluorine will be. Which of the following set of quantum numbers incorrect for last electron of fluorine atom. The optimum electron configuration of the 2P orbital comprises 6 electrons so since Fluorine is so near perfect electron configuration the electrons are held very tightly to.
N 1 l 0 m l 0 and m s 12. Eqn 1 l. The positions of the atoms inside the unit cell are described by the set of atomic positions x i y i z i.
It indicates the number of shells. N2 l 1 m 0 s 12. 2 ℓ 0 1 2.
The electron is located on the second energy level in the 2p subshell in the 2pz orbital and has spin-down. The upper limit of our atmospherea. 1 n 1 2 3 and so on.
Then for the third electron which is the first one in the L shell n 2 so l may be 0 or 1 m l may be -1 0 1 and for each of those m s may be 12 or -12 etc. Correct answer to the question G The set of quantum numbers for the 6th electron in fluorine atom. As a result an atom of fluorine should contain 9 electrons.
Among all occupied atomic orbitals 2p is the one of the highest potential energy. Quantum numbers are a set of 4 imaginary numbers which explain the position and spin of electrons in an atom it can not explain an atom as a whole Iodine has 53 electrons so there are 53 sets of. The values of the four quantum numbers for each of the nine electrons of fluorine are.
Azimuthal Quantum number It is denoted by l. Element Atomic Number Element Symbol Element Name Element Element Quantum Numbers 1. List the following sets of quantum numbers in order of increasing energy.
They have been determined from a study of nature. 2 He - Helium. What is the quantum number following the numbering conventions that describes the electron that was gained by fluorine.
N - 1 3 m ℓ starts at negative ℓ runs by whole numbers to zero and then goes to positive ℓ. Hence the sixth electron of fluorine is found in the 2p subshell where. Which of the following set of quantum numbers incorrect for last electron of fluorine atom.
It is the magnetic quantum number and it. A 30 0 - B 31 0 - C 3 1 1 D 2 1 0 - Na 3tra T 36274 FTTH foru alich vielli 1 HRT ZTE A 30 0 - B 3 1 0 - C 311 1 D 2 1 0 -5. Be explicit if you have questions.
Vanadium and the four Quantum numbers. Element 9 of Periodic table is Fluorine with atomic number 9 atomic weight 189984032. The rules ARE NOT just any old arbitrary ones.
Fluorine symbol F has a Base Centered Monoclinic structure and Colorless color. Re where space shuttle flies and where aurora can be founda. What are the four quantum numbers of vanadium.
The four quantum numbers the principal quantum number n the angular momentum quantum number ℓ the chemistry Given the shell model of the atom suggest a possible reason that Lewis proposed a maximum of two electrons for hydrogen and a maximum of eight electrons for carbon nitrogen oxygen and fluorine. N 4 l 0 m l 0 m s 12. B n 2 ℓ 1 m ℓ 0 m s -12.
It is the layer of atmosphere where jet and aircraft fly Stratosphere B. I principal quantum number n ii orbital quantum number l iiiMagnetic quantum number m iv Spin quantum number s The electronic configuration of fluorine is. N 1 l 0 m l 0 and m s -12.
He 2s2 2p5 H e 2 s 2 2 p 5. It is the layer of atmosphe. Ml is the third quantum number.
Electronegativity of Fluorine. It is the angular momentum quantum number and refers to the shape of the orbital. Fluorine is the 9 th element on the periodic table and is located in the p block at the right end of the second period.
Which one of the following sets of quantum numbers is not possible. The correct set of quantum numbers among the given options is. The correct set of quantum numbers for the unpaired electron of F atom is2 0 0 123 1 1 122 1 1 122 0 0 12The electronic configuration of fluorine is 1s Grade.
The correct set of quantum numbers for the last electron in Na ion is. The ground state electron configuration for the fluorine atom is. N 2l 1ml 0ms 1 2.
Principal Quantum number It is denoted by n. 3 1 -1 ½ ie correct option is 3. N3 l1 m1 or 0 or -1 s½ or -½.

Fluorine Can Gain An Electron To Become Fl Clutch Prep
Solved Write A Complete Set Of Quantum Numbers For Each Of Chegg Com

Which Of The Following Set Of Quantum Numbers Incorrect For Last Electron Of Fluorine Atom Youtube
Solved 4 Identify The Highest Energy Electron S In Each Of Chegg Com
Solved 6 Predict All Of The Quantum Numbers 4 Of Them For Chegg Com