Hence the correct set of quantum numbers is. L 0 up to n-1.
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium
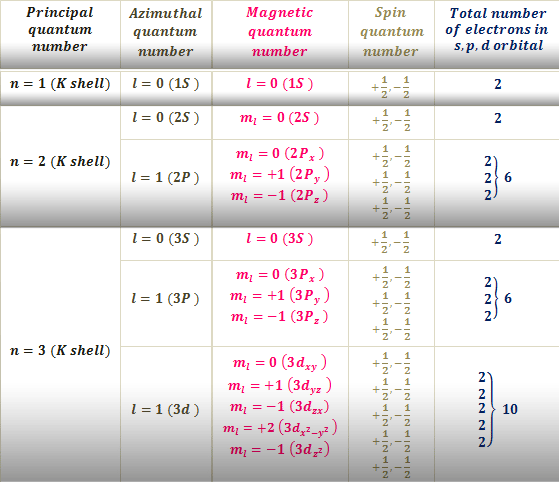
First - Primary Quantum number n size of electron cloud n 1 up to in reality n 1 -7 Second Azimuthal or Angular Momentum Quantum number l shape of electron cloud.

How to find the set of quantum numbers for an element. 119 rows Click on Element Atomic Number Element Symbol Element Name and. Complete step by step answer. M0 since were still in the s sub shell only and s12 or s-12 arbitrarily assigned values note12 or -12 are used to distinguish between electrons as no two electrons can have the same electronic configuration but here the question isnt that specific so we need not bother.
N3 l1 m1 or 0 or -1 s½ or -½. Value is a guess based on periodic table trend. This quantum number can only be positive non-zero and integer values.
Assign a correct set of four quantum numbers for the valence electron in a sodium atom. Value is a guess based on periodic table trend. N l ml ms.
The values of four quantum numbers of valence electron of an element are n4 l0 m0 and s12. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12. This question has multiple correct options.
An atom with an nth electron shell can hold 2n2 electrons which is the first shell that can hold 2 electrons the. This number determines the size and energy of the orbital. From the quantum numbers we can say that the last electron enters 3p orbital.
Quantum Numbers and Atomic Orbitals By solving the Schrödinger equation Hψ Eψ we obtain a set of mathematical equations called wave functions ψ which describe the probability of finding electrons at certain energy levels within an atom. Each electron in an atom has a unique set of quantum numbers. The correct set of quantum numbers among the given options is.
1 Sodium has a total of eleven electrons and one of them is the sole valence electron that sodium has. One of the electrons has the quantum number set 2 1 -1 12 and the other has the quantum number set 2 1. Identify the wrong set of quantum numbers from the following.
3 1 -1 ½ ie correct option is 3. The element is a transition metal. Quantum numbers are important because they can be used to.
I propose to assign all ten sets of quantum numbers and build up to the eleventh set which will be the answer to the question. Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Principal Quantum Number n The principal quantum number n indicates the shell or energy level in which the electron is found.
How to find elements given quantum numbers by Jerry Zhao - June 12 2012. Find the principal number which denotes the elements energy by looking in which period the element is found. This video provides 3 example practic.
Angular Momentum Quantum Number l associated with the number of different types of subshells in. Value is a guess based on periodic table trend. By taking the given values and using these formulas derive the electronic configuration and find out the atom.
The principal quantum number can be determined by looking at the period numbered row of the element on the periodic table. The value of n can be set between 1 to n where n is the value of the outermost shell containing an electron. For example sodium is in the third period of the table so its principal quantum number is 3.
Value is a guess based on periodic table trend. State the four quantum numbers and the possible values they may have. There are four different quantum numbers.
Two electrons that are found in the same atom have different quantum number sets. Notes on the Quantum Numbers of particular elements. This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion.
According to the Pauli Exclusion Principle no two electrons can share the same combination of four quantum numbers. This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. A wave function for an electron in an atom is called anatomic orbital.
For example an Iodine atom has its outmost electrons in the 5p. This set of quantum numbers represents the last electron to be added to complete the ground state electron configuration of an element. The quantum number l with value 1 corresponds to p orbital.
Value is a guess based on periodic table trend. The principal quantum numbers of electrons in the S-block and P-block. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that.
Which is the correct set of 4 quantum numbers. Quantum numbers are n 3 l 1 m l 0 m s 1 2. Principal Quantum Number n has integral values 1 2 3.
The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. The set of quantum numbers n 4 l 0 m 0 s dfrac12 corresponds to the most loosely bound ground state electron of which one of the following atoms A Na B Cl C Cr D Rb. The quantum numbers of Lithium are written as n12l0ml0ms1212 n 1 2 l 0 m l 0 m s 1 2 1 2.
What are the 4 quantum numbers for lithiums first electron. Electron shells are a set of feasible states that have the same principal quantum number n the number before the letter on the orbital that the electron can occupy.

Orbitals Quantum Numbers Electron Configuration Multiple Choice Practice Problems Youtube

Quantum Number Orbital Definition Formula Diagram Shape
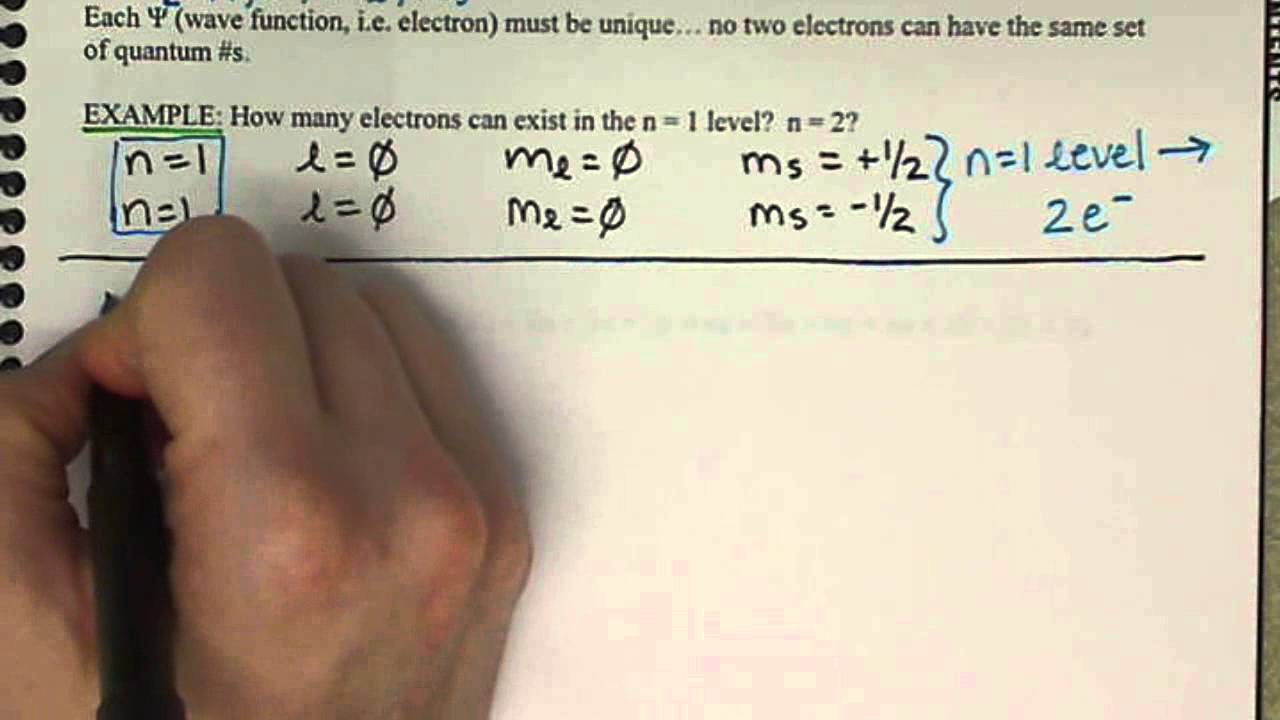
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube

Teach Students How To Find Quantum Numbers With This Worksheet Printable It Simplifies The Process Chemistry Classroom Teaching Chemistry Chemistry Activities
What Will Be The Quantum Numbers For The Last Electron Of Sodium Atom Quora