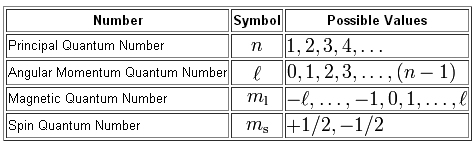
0 ℓ n 1. The direction of spin is described by spin quantum number.

How To Determine The Maximum Number Of Electrons Using Allowed Quantum Numbers 8 Cases Youtube
This video shows you how to determine or calculate the maximum number of electrons using allowed quantum numbers n l ml and ms.
How to determine ms in quantum numbers. It is the number of subshells per principal energy level. If l 2 mcan be either -2 -1 0 1 or 2. N 2 l 1 m l 0 m s 1 2.
Ms 12 for spin up and ms -12. Spin Quantum Number m s.
What is the symbol for spin quantum number. Principal quantum number. When ℓ 3 there are seven permissible mℓvalues.
L 2 f. The second quantum number is l telling the subshell in which electron is present. The three coordinates that come from Schr dingers wave equations are the principal n angular l and magnetic m quantum numbers.
The mℓvalue of 5 is not permitted in this set of quantum numbers. It covers about 8 cases. 3 2 1 0 1 2 3.
Ml 2l 1. S m s s. It is either positive or negative one half.
Orbitals that have same value of principal quantum number form a Shell n. L 0 p. The electron in an atom not only moves around the nucleus but also spins about its own axis.
Msis allowed to only be values of 12or 12. The value of m can be from -l to l. Quantum numbers arent used to determine the number of electrons on an atom- the number of electrons on a neutral lone atom are of course determined by the number of protons and the quantum numbers of the electrons are just a.
Which of the four quantum numbers NL ml MS determine. The Pauli exclusion principle Wolfgang Pauli Nobel Prize 1945 states thatno two. L 0 n-1 determines the shape of an orbital.
The magnitude spin quantum number of an electron cannot be. For any l the magnetic quantum number can take on integer values from l to l. The magnetic quantum number m can be any integer between -land l.
Example 5A hydrogen atom has n 5 and mℓ 2. For example if l0 ml0 and if l1 ml can equal -1 0 or 1. In order to determine the ms value for an elements last valence electron start numbering from left to right in the subshell of the element beginning with the -l value and progressing to the l.
The value of the m is depending upon the value of l. L 1 d. Ml is magnetic quantum number and refers to the number of orbitals per subshell.
Azimuthal Quantum Number l. The magnetic quantum number m can be any integer between - l and l. Answer 1 of 2.
7 In f compare the mℓto the ℓ value. An electron can spin in only one of two directions sometimes called up and down. The first quantum number is n telling the shell in which the electron is present.
Its orientation in space. The spin quantum number tells us the orientation of an electron within an orbital and has two possible values. This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence.
The number of the orbitals for n. Magnetic quantum number projection of angular momentum m ℓ. Hence there are 2l1 different orientations for each subshell.
If you add up all the electrons in the shell n5 you will get a total of 50 electrons. This question is entirely backwards. If l 2 m can be -2 -1 0 1 or 2.
Describe the allowed combinations of the n l and mquantum numbers when n 3. Ms 12 for spin up and ms -12 for spin down. C Shape of an orbital.
N 2 l 1 m l 0 m s 1 2. It is determined by the Spin Quantum Number ms. The spin quantum number tells us the orientation of an electron within an orbital and has two possible values.
Shells and Subshells of Orbitals. M_l is the magnetic quantum number corresponding to the projection of the angular momentum of an orbital ie. Half of these electrons are spin up ms12 and half these electrons are spin down ms-12 so the maximum number of electrons that can have the quantum numbers of n5 and ms12 will be 25 electrons.
As the symbol suggests it has to do with l the angular momentum quantum number. M s ½ or -½. Orbitals within the shells are divided into subshell l s.
The magnetic quantum number denoted by m. L can take values from 0 to n-1. That means-If l 0 then m 0.
The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. Determine the number of electrons using quantum numbers by first counting the number of electrons in each full orbital based on the last fully-occupied value of the principle quantum number then adding the electrons for the full subshells of the given value of the principle quantum number and then adding two. This video provides 3 example practic.
Since the spin can be 12 or 12 there are two combinations. This number gives the information about the direction of spinning of the electron present in any orbital. Click here to check your answer to Practice Problem 7.
E Orientation of spin of the electrons. Ms is spin quantum number and refers to spin of electron. We can define the magnetic quantum number as the quantum number which specifies the orientation of the orbitals of a subshell in three-dimensional space.
Azimuthal quantum number angular momentum ℓ. Ms ½ or -½. The Magnetic Quantum Number ml describes the configuration of the orbitals according to a set of axes in three dimensions.
The spin angular momentum is an intrinsic property like rest mass and charge. Specifies the orientation of the spin axis of an electron. These quantum numbers describe the size shape and orientation in space of the orbitals on an atom.
Quantum Numbers Introduction To Chemistry

Solved Four Quantum Numbers The Principal Quantum Number Chegg Com

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube

Distribution Of Electrons Quantum Numbers Mcc Organic Chemistry

Quantum Numbers The Easy Way Youtube