Value is a guess based on periodic table trend. This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence.

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
Heres the rule for m ℓ again.
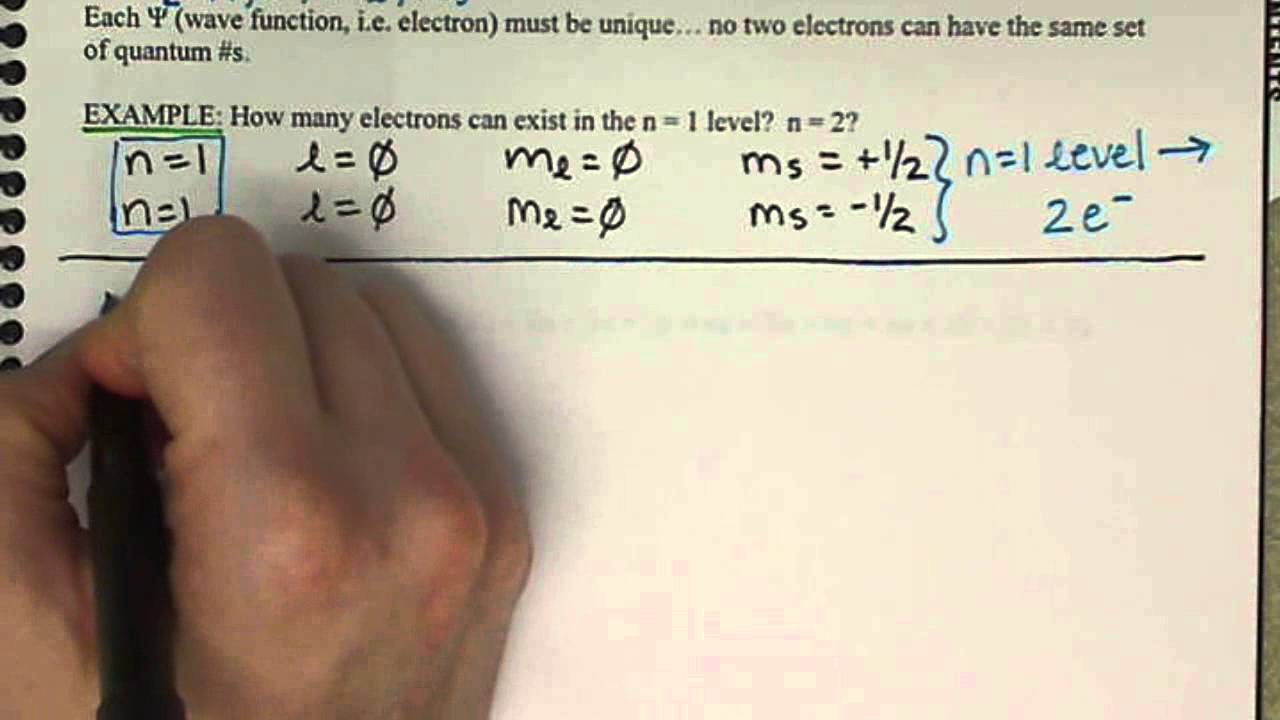
How to write quantum numbers for elements. The last quantum number is the spin quantum number. S m s s. We have three orbitals we have three p orbitals here one for each axis.
We could write a pz orbital here and then this one right here would be a py orbital. Value is a guess based on periodic table trend. The lowest possible value of l is 0 and its highest possible value depending on the principal quantum number is n - 1.
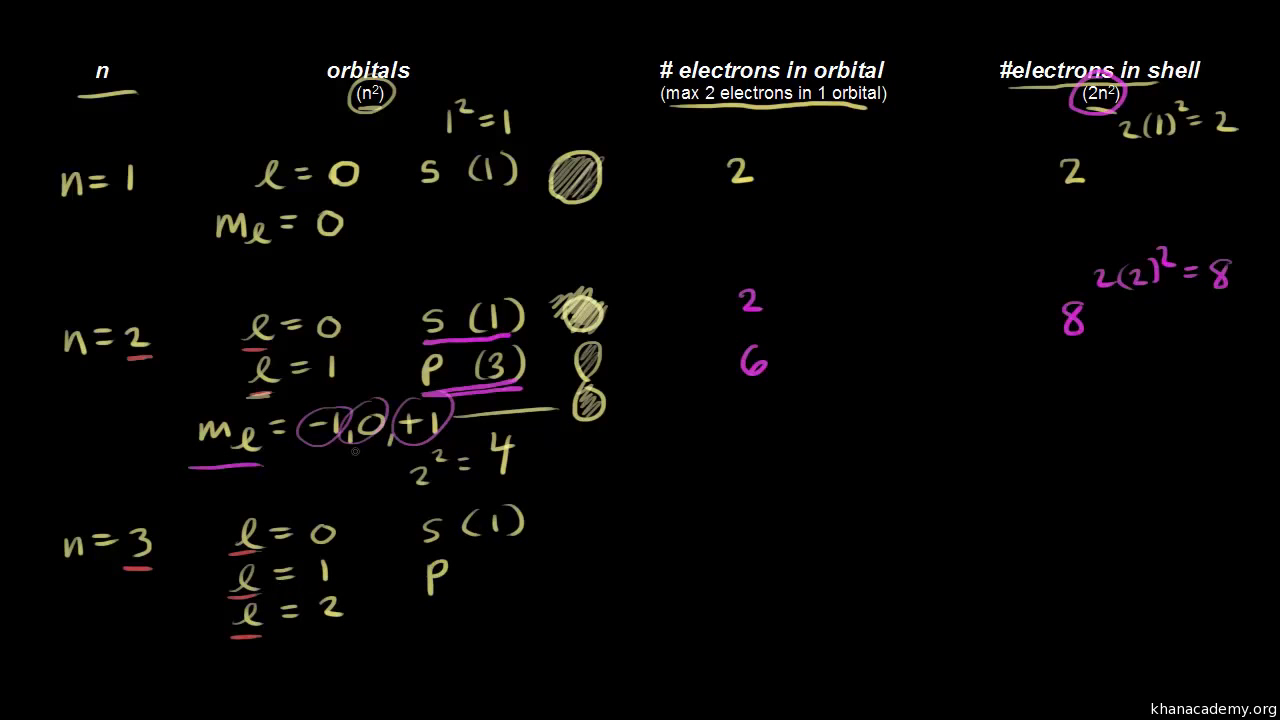
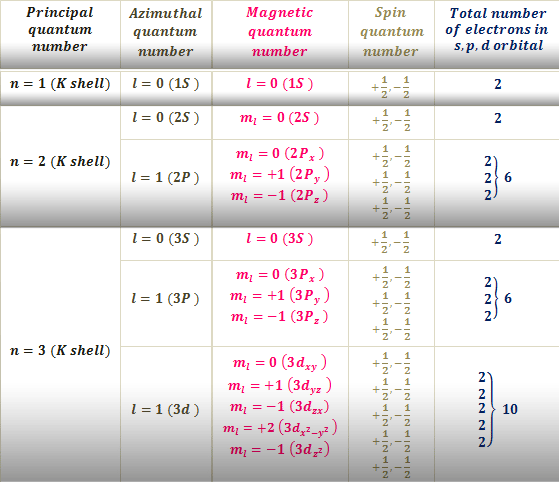
The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. 119 rows This Quantum Numbers chart table gives the Quantum Numbers of all the elements of periodic table. Magnetic quantum number projection of angular momentum m ℓ.
Electron configurations are written using the principal quantum number n followed by the orbital s p d or f with the total number of electrons written as a superscript. The angular momentum quantum number l also referred to as the secondary quantum number or azimuthal quantum number describes the shape of the orbital that an electron occupies. The spin quantum number is m sub s here.
N Pricipal Quantum Number. Show activity on this post.
1s 2 For writing ground state electron configurations a few main steps should be followed. Lets go to the last quantum number. Each electron in an atom is described by four different quantum numbers.
Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Get the quantum numbers from the differential electron the last one that fills the orbital. QUANTUM NUMBERS WORKSHEET 1.
Value is a guess based on periodic table trend. For example sodium is in the third period of the table so its principal quantum number is 3. 0 ℓ n 1.
Click on Element Atomic Number Element Symbol Element Name and Element Quantum Numbers headers to. For instance lets take a look at carbons electron configuration. State the four quantum numbers then explain the possible values they may have and what they actually represent.
Can be 1 to 7 l Secondary Quantum NumberOrbital Shape Quantum number. Causey shows you step by step how to write quantum numbers for specific electrons in certain elements. This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion.
Since the spin can be 12 or 12 there are two combinations. Value is a guess based on periodic table trend. Quantum numbers and shells Filling the shells Relationship with the periodic table How to write electron configuration.
Write the electron configuration. This video provides 3 example practic. N is 3 and l is 2 which corresponds to d so the last shell is 3 d 1 therefore we have 21 e and the answer will be S c ion from the periodic table.
The magnetic spin quantum number relates the spin of the electron per orbital. N 1 2 3 8. Determine ml by labeling the block from -l to l.
Identify the quantum numbers of the given elements. 2 on a question Activity 4. Find the principal number which denotes the elements energy by looking in which period the element is found.
All you need to know are. N 2 l 1 m l 0 m s. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12.
Write your answer on a separate sheet of paper. Quantum numbers and shells. To get the quantum numbers you just have to follow 2 simple steps.
This would be a pz orbital. How are quantum numbers derived. Since ℓ 1 we start with -1 go to zero and end up at 1.
Principal Quantum Number n. The electron configuration indicates the distribution of electrons in the electron shell of an atom to different energy states orbitals. Notes on the Quantum Numbers of particular elements.
This gives us three values for m ℓ when ℓ 1. The magnetic quantum number relates the spatial orientation of the orbital and ranges from ll ms. Hopefully you can see that since m s takes on ½ and -½ we will wind up with six sets of quantum numbers.
If the quantum numbers of an ion are given how can we identify this ion by its quantum numbers. Put all of the information. Learn about atomic orbital the four quantum numbers principal angular momentum magnetic and spin and how to write quantum numbers based on electron configuration.
Determine ms by determining if the element is in the first or second half of the block. We start with step 1 write the electron configuration. M s ½.
Represents the energy level the electron is in linked to the periods of the periodic. Principal quantum number. Start at negative ℓ run by whole numbers to zero and then go to positive ℓ.
What I have so far. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one.
Azimuthal quantum number angular momentum ℓ. M l 0.

Quantum Number Orbital Definition Formula Diagram Shape
Quantum Numbers For The First Four Shells Video Khan Academy
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium

Quantum Numbers The Easy Way Youtube

How To Write Quantum Numbers For Electrons Youtube