Because angular momentum is a vector the Spin Quantum Number s has both a magnitude 12 and direction or -. M s ½ or -½.

6 5 Assigning Quantum Numbers To Cr Youtube
The spin angular momentum quantum of an electron is.
How to assign spin quantum numbers. The spin quantum number is used to identify the spin of the electron which can be either 12 or -12. In order to assign the various quantum numbers we must follow some rules. They are independent variables except that the overall wave functions for identical particles must satisfy the antisymmetrization requirements.
Layer s - l 0. In the early 1920s Otto Stern and Walther. One electron can be spin up and the opposite electron is spin down.
As we are able to see in a single orbital the orientation of the 2 electrons is at all times the other of one another. Solved Examples for You. One electron will be spin up and the other electron is spin down.
This number depends on the last layer that has been filled. N 3 l 1 m_l. If the last electron that enters is spin up then ms 12.
For example you can have. The Pauli exclusion principle Wolfgang Pauli Nobel Prize 1945 states thatno two. Denoted as m_s the electron spin is constituted by either upward m_s12 or downward m_s-12 arrows.
This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion. This will also be the case for the spin coordinates. To calculate it draw electronic structure distribute the electron according to Hunds rule then the up arrow show ½ and dow error show -½ example.
Spin Quantum Number is denoted by the symbol s. Thus the last electron is single. An electron spins around an axis and has both angular momentum and orbital angular momentum.
It can have about only two values ie. The state of an electron in a hydrogen atom can be expressed in terms of five quantum numbers. Adding the Spins of Two Electrons The coordinates of two particles commute with each other.
Therefore total of 8 electrons that can share those two quantum numbers. Spin Quantum Number represents the direction of the spin of the electrons. Since the spin can be 12 or 12 there are two combinations.
In atomic physics the spin quantum number is a quantum number which describes the intrinsic angular momentum of an electron or other particle. Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions sometimes called up and down.
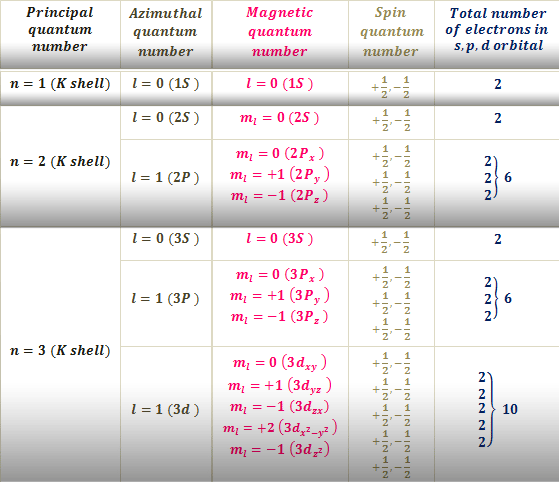
The name comes from a physical spinning of the electron about an axis. For example if the electron configuration ends in 4s 2 the principal quantum number will be 4. L Proposed by Sommerfeld to explain the hyperfine splitting in atomic spectrum a It tells us about iThe number of subshells present in a given orbit.
Beginalign textspin-1 times spin 12. The spin angular momentum projection quantum number is m s spin up or spin down. Finally the spin quantum number m_s which can only take two possible values gives you the spin of the electron.
If the final electron that enters is spin down then the ms -12. Ii Relative energies of the subshells. Iii Shapes of orbitals.
Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one. The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. The other three are the principal quantum number azimuthal quantum number and magnetic quantum number.
We consider the combinations that are eigenstates of total angular momentum and the way to find them is as the first answer stated. Spin quantum number is the fourth quantum number which describes the orientation of the electron spin rotation in space. The Spin Quantum Number m_s describes the angular momentum of an electron.
Electron Spin or Spin Quantum Number is the fourth quantum number for electrons in atoms and molecules. This can either be in the direction of clockwise or even anti-clockwise. Pair the first and second spin into a spin 1 triplet and a spin 0 singlet and then using Clebsch-Gordan coefficients take their products with a doublet.
And the azimuth quantum number sub orbital value will be ln-1 01 s p Now the magnetic quantum number is m -l0l -1 0 1 Finally we know the electron distribution 1S2 2S2 2P6 3S1 Hunds Rules. 8 O 16 No of electrons is 8. The idea of spin was originated from the Stern-Gerlach experiment.
N 2 l 1 m l 0 m s. Now notice that the values the magnetic quantum number can take depend on the value of l the angular momentum quantum number. An electron is in one of the 3d orbitals.
The fine and hyperfine structures of the hydrogen spectrum are explained by magnetic interactions within the atom. If the final electron that enters is spin up then ms 12. This number coincides with the number of the last level of the electron configuration.
You can form several possible sets of quantum numbers to describe two electrons located in 3p orbitals. Maximum number of orbitals n2 in a given orbit 2 Angular Momentum or Azimuthal Quantum No. If the last electron that enters is spin down then the ms.
See full answer below. 3 times 2 4 2 textspin-0 times spin 12. The most important rule is that there is no probability of locating two.
SImply put two electrons of opposite spins can share one orbital given by ml1 per subshell given by l. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12. Spin Quantum Number m s.
The phrase was originally used to describe the fourth of a set of quantum numbers which completely describe the quantum state of an electron in an atom. Layer p - l 1. The four sets of quantum numbers principal azimuthal magnetic angular momentum and spin are frequently utilized in order to describe a.
The spin quantum number is one of the four quantum numbers that identify an electron in an atom.

Quantum Numbers Chemistry Socratic
Phoenix Learning Center Atomic Structure Quantum Numbers At The General Chemistry Level Regents Level The Only Quantum Number That Is Required To Know Is Quantum Number N N Represents A Specific

Question Video Determining The Number Of Electrons Using Two Quantum Numbers Nagwa

Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium

Quantum Numbers The Easy Way Youtube
