The quantum numbers for electrons are determined by the rules for quantum numbers. Value is a guess based on periodic table trend.
The principal quantum number n that can take on values 1 2 3.
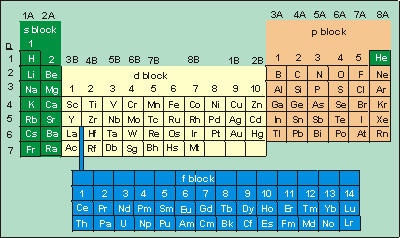
How to determine quantum numbers from periodic table. L describes the shape of the orbital. Look at following examples for better understanding. Thus period of S is 3.
The principal quantum number can be determined by looking at the period numbered row of the element on the periodic table. For example sodium is in the third period of the table so its principal quantum number is 3. Value is a guess based on periodic table trend.
Value is a guess based on periodic table trend. By 2ℓ 1 rule the value of magnetic quantum number m. Look at the Periodic Table of Elements and find the element that you want to know the quantum number for.
Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Value is a guess based on periodic table trend. Quantum numbers and the Periodic Table - YouTube.
Notes on the Quantum Numbers of particular elements. Lets look at various values of l and their corresponding m_l. The blocks match the the angular momentum quantum number ℓ.
Since the arrangement of the periodic table is based on the electron configurations Figure PageIndex4 provides an alternative method for determining the electron configuration. From solving the SE. Value is a guess based on periodic table trend.
This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion. Orbitals and the Periodic Table Ram Seshadri MRL 2031 x6129 seshadrimrlucsbedu. For example sodium is in the third period of the table so its principal quantum number is 3.
Using the Periodic Table to Determine Quantum Numbers. 1s 2 2s 2 2p 63 s 23 p 4 3 is the highest energy level of electrons or principal quantum number. 1s 2 2s 2 2p 6 3s 2 3p 64 s 2 3d 4 4 is the highest energy level of electrons or principal quantum number.
Thus period of Cr is 4. It is easy to find group no period no as well as block of an element. Answer 1 of 9.
Periodic tables are broken down into blocks that are based on the subsidiary quantum number 𝑙 and rows that are based on the principal quantum number 𝑛. Aufbaus not Bohrs 1. The only info you will need is the configuration of the element.
Value is a guess based on periodic table trend. L 0 - m_l 0 orbital s l 1 - m_l -101 orbital p l 2 - m_l -2-1012 orbital d l 3 - m_l -3-2-10123 orbital f and so on. Value is a guess based on periodic table trend.
Value is a guess based on periodic table trend. Be sure to only include orbitals allowed by the quantum numbers no 1p or 2d and so forth. As we will see the principal quantum number corresponds to the row number for an atom on the periodic table.
Value is a guess based on periodic table trend. Finally draw diagonal lines from top to bottom as shown. There is a section of the periodic table that corresponds to the 1s atomic orbital and sections that correspond to the other atomic orbitals such as the 2s and 3d atomic orbitals.
The principal quantum number n specifies the row within the periodic table of the highest shell valence electrons. The value of azimuthal or orbital quantum number ℓ 2 for d orbital. For a 4d orbital three quantum numbers are associated as.
For every atom these quantum numbers would be used for the first ten electrons because they represent the lowest ten energies for electrons. Figuring out how to obtain a periodic table is just not difficult but understanding how the elements constitute this helpful and interesting piece of expertise requires a bit of a science backdropNew Available Periodic Table Quantum NumbersThe periodic tables were developed so that scientists could easily determine the atomic dumbbells of numerous elements. For hydrogen-like atoms one finds that electrons in many-electron atoms are completely described by a set of four quantum numbers.
Angular Momentum Quantum Number left l right The angular momentum quantum number signified by l describes the general. Find the principal number which denotes the elements energy by looking in which period the element is found. Find the principal number which denotes the elements energy by looking in which period the element is found.
The principal quantum numbers of electrons in the S-block and P-block. The value of principal quantum number n 4 because it says itself. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that.
As the symbol suggests it has to do with l the angular momentum quantum number. The different types of orbital are specified by the azimuthal quantum number l. Notes on the Quantum Numbers of particular elements.
Value is a guess based on periodic table trend. 119 rows This Quantum Numbers chart table gives the Quantum Numbers of all the. If playback doesnt begin shortly try restarting.
To find period number Find the highest number in the electronic configuration of the element. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12.
Chemistry Life The Universe And Everything

What Are The Quantum Numbers For Beryllium Study Com

Solved Relating Quantum Numbers And Electron Configurations Chegg Com