The principal quantum number n describes the energy of an electron and the most probable distance of the electron from the nucleus. SECONDARY QUANTUM NUMBER l - Represents the energy sublevel or type of orbital occupied by the electron.

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
Since electrons are in s -orbital.
How to find the 4 quantum numbers of an electron. Electrons can be situated in one of three planes in three dimensional space around a given nucleus x y and z. An atom consists of a large number of orbitals which are distinguished from each other on the basis of their shape size and orientation in space. For 8 t h electron of oxygen atom the four quantum numbers are n 2 l 1 m 1 or 1 s 2 1 or 2 1.
The 15 electrons of the phosphorus atom will fill up to the 3 p orbital which will contain three electrons. 425 484 Views. The Principal Quantum Number.
The first quantum number describes the electron shell or energy level of an atom. L is the angular momentum quantum number which is 0 for s 1 for p your case 2 for d 3 for f 4 for g. There are two 3s electrons and two 3p electrons.
It is always a positive integer that is n 1 2 3. Ms s s 1 s 2 s 2 s 1 s. For facts physical properties chemical properties structure and atomic properties of the specific element click on the element symbol in the below periodic table.
N is the shell number which you indicated to be 3. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The last electron added is a.
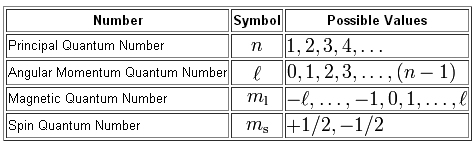
Maximum number of electrons an orbital can have is 2. Answer 1 of 6. Quantum numbers These four quantum numbers are used to describe the probable location of an electron in an atom.
In the below periodic table you can see the trend of Quantum Numbers. The order of filling of the energy levels is 1 s 2 s 2 p 3 s 3 p 4 s. In general the values of ms range from s to s where s is the spin quantum number associated with the particles intrinsic spin angular momentum.
Electrons in the same atom that have the same principal quantum number are said to occupy an electron shell of the atom. For a given value of the angular momentum quantum number l there can be 2 l 1 values for m l. The principal quantum number can be any nonzero positive integer.
The first quantum number describes the electron shell or energy level of an atom. For electrons in 4s orbital. Normally we use the symbols n l m and s lower case and italicized to describe the quantum numbers for an electron in an atom.
Energy n angular momentum ℓ magnetic moment mℓ and spin ms. An electron has spin number s 1. To completely describe an electron in an atom four quantum numbers are needed.
The 8th electron in the oxygen atom belongs to 2p subshell. For l 0 m l 0. The atomic number of phosphorus is 15.
This is a very fundamental topic as the entire chemistry lies on the basis of electrons and their participation in a reaction. PRINCIPAL QUANTUM NUMBER n - Represents the main energy level or shell occupied by an electron. Keeping this in consideration what are the 4 quantum numbers describe each.
In atoms there are a total of four quantum numbers. The first quantum number describes the electron shell or energy level of an atom. For l 1 m l 1 0 1.
The principal quantum number largely determines the energy of an electron. The answer is 4. For both the electrons in an orbital first 3 quantum numbers are same.
The first quantum number is called the principal quantum numbern. Since electrons are in 4th shell. For electrons located in a 3p orbital the principal quantum number is equal to n3the third energy level So you know that you have n3.
All start with 3 so all will have a principal quantum number of 3. The principal quantum number of electrons in the D-block is period minus 1. To completely describe an electron in an atom four quantum numbers are needed.
The principal quantum number of electrons in the F-block is period minus 2. 11 Votes To completely describe an electron in an atom four quantum numbers are needed. L 0 1 2.
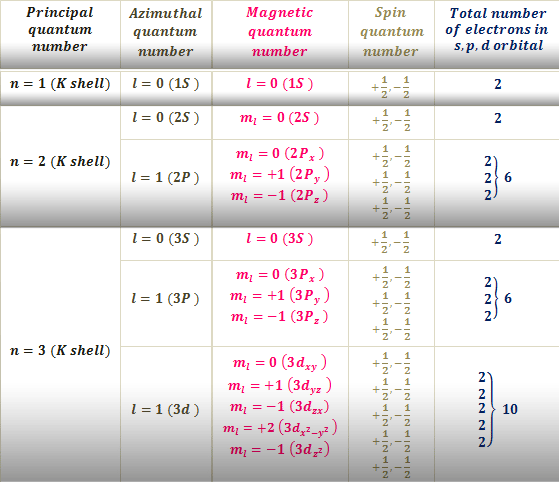
Periodic Table of Elements with Quantum Numbers Trends. Angular Momentum Quantum Number left l right The angular momentum quantum number signified by l describes the energy sublevel subshell and general shape or region an electron occupiesits orbital shapeThe value of l depends on the value of the principal quantum number n. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that can occupy the orbital.
Thus a phosphorus atom contains 15 electrons. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence.
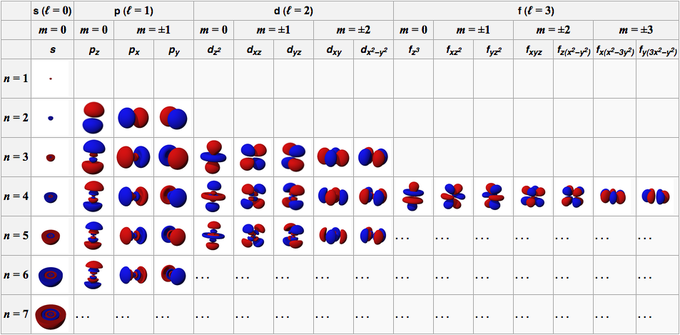
Click to see full answer. Every value of l corresponds to a different orbital shape. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s.
Quantum numbers are actually a set of four numbers which helps in determining the complete information about the location of electrons in an atom. The angular momentum quantum number can have positive values of zero to left n-1. 2 consequently ms will be 1.
1 2 3 4. The principal quantum number n the orbital angular momentum quantum number l the magnetic quantum number m l and the electron spin quantum number m s. Here we have l 0 which corresponds to the s orbital shape l 1 corresponds to the p orbital shape and l 2 the d.
L 0 or 1. The orbital characteristics are used to define the state of an electron completely and are expressed in terms of three numbers as stated Principal quantum number Azimuthal quantum number and Magnetic quantum number and Spin. This video provides 3 example practic.
Next find the value of the angular momentum quantum number l which gives you the subshell in which the electron is located. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12.
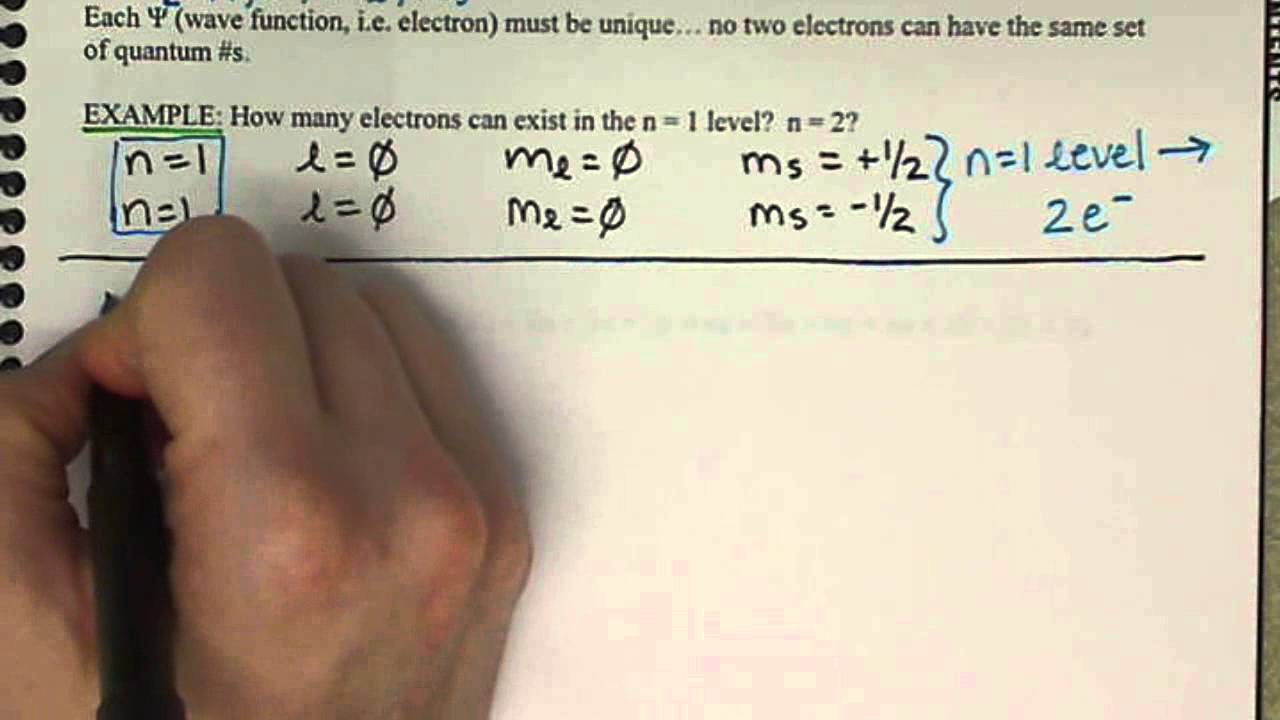
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium

Quantum Numbers Introduction To Chemistry

Orbitals Quantum Numbers Electron Configuration Multiple Choice Practice Problems Youtube
Quantum Numbers Introduction To Chemistry