However boundaries or constraints need to be put in place to match the equations with the physical world. For the element Nickel how many ground state electrons will have the quantum numbers n3 and ml -1.
Quantum Numbers Video Quantum Physics Khan Academy
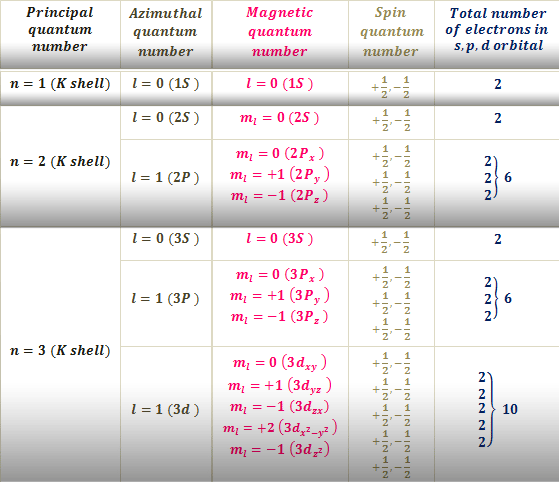
Principal quantum number n.

How to find magnetic quantum number of an element. Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. L 1 d. Principal quantum number azimuthal quantum number magnetic quantum number and spin quantum number.
It indicates the relative distance of electrons having different n values in multi-electron atom. Spin Quantum Number s-12. 119 rows Mouseover on the chart to see the element name and Quantum Numbers of.
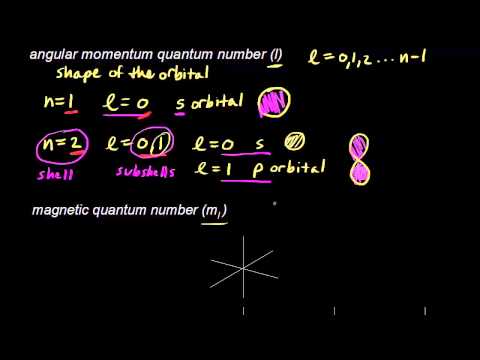
N 2 n 1. Secondary quantum number - l 2. Determine ml by labeling the block from -l to l.
As the symbol suggests it has to do with l the angular momentum quantum number. Notes on the Quantum Numbers of particular elements. Magnetic Quantum Number m1.
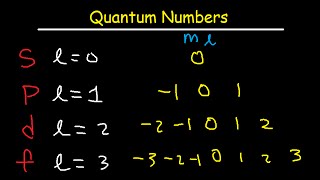
Answer 1 of 3. The number of orbitals in a subshell is equivalent to the number of values the magnetic quantum number ml takes on. Magnetic quantum number Spin quantum number.
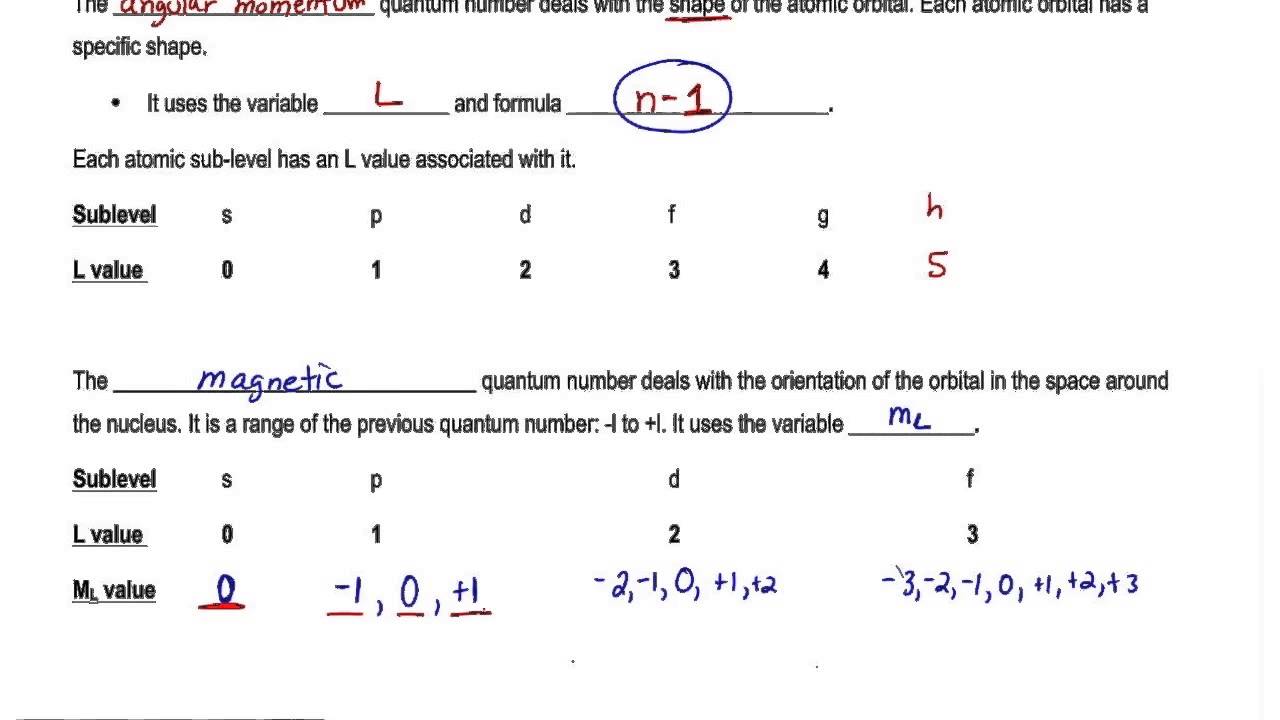
The magnetic quantum number m l depends on the azimuthal quantum number l which relies on the principal quantum number n. A helpful equation to determine the number of. Find the principal number which denotes the elements energy by looking in which period the element is found.
Orbitals within the shells are divided into subshell l s. Value is a guess based on periodic table trend. The magnetic quantum number can be derived from solving the azimuthal equation of the hydrogen Schrodinger equation.
When n 3 l 0 l 2 or 3s 3p and 3d-orbitals. Value is a guess based on periodic table trend. Permitted values of l for a given value of n have 0 to n-1.
L 0 p. Value is a guess based on periodic table trend. This video provides 3 example practic.
The value of l is dependent on the value of n l 0 1 2 n. The magnetic quantum number m can be any integer between -l and l. Principal quantum number n n 1 2 3.
Heres what I did but Im not sure if Im right. Give the possible values of n l and ml for this electron. Azimuthal Quantum Number l1.
Orbitals that have same value of principal quantum number form a Shell n. Azimuthal Quantum Number l1.
An electron is in one of the 3d orbitals. Magnetic Quantum Number m1. If n 3 for example l can be either 0 1 or 2.
When n 1 l 0 or 1s-orbital. Quantum numbers in general and magnetic quantum numbers in particular can be derived while solving the Schrodinger equati on. For the 3d orbital Principal quantum number n 3.
Together they describe the unique quantum state of an electron. They can only be identified by the spin quantum number. The angular quantum number l can be any integer between 0 and n - 1.
Value is a guess based on periodic table trend. For example sodium is in the third period of the table so its principal quantum number is 3. Therefore the magnetic quantum numbers l 0 1 2 3.
The magnetic quantum number distinguishes the orbitals available within a subshell and is used to calculate the azimuthal. Spin Quantum Number s12. Spin quantum number - s -½.
It indicates the value of energy level and the order n 1 2 3 and so on but not zero. But the total number of different values of l equal to n. M_l is the magnetic quantum number corresponding to the projection of the angular momentum of an orbital ie.
How to find Magnetic Quantum Number. Its orientation in space. For phosphorus Z 15 Therefore Electronic configuration would be 1s² 2s² 2p⁶ 3s² 3p³ 15th electron is present in 3pz orbital Quantum numbers are 1 Principal quantum number n 3 2 Azimuthal quantum number l 1 3 Magnetic quantum number ml 101 4Spin q.
It determines the total energy En of an atom and ions containing one electron. Cr 24 Rb 37 Br 35 Lu 71 Au 79. The magnetic quantum number is one of four quantum numbers in atomic physics.
Azimuthal quantum number angular momentum ℓ for n 3. But for n 2 l 0 1 or 2s 2p-orbitals. So for n3 Nickel there are 16 electrons in the ground state yes.
This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. Its location is further narrowed down by the angular momentum quantum number l which tells us the subshell and its general shape. Magnetic quantum number ml 2 1 0 1 2.
Determine ms by determining if the element is in the first or second half of the block. And then to find the magnetic quantum number I must first find l. Mℓ 2 1 0 1 2.
The magnetic quantum number m can be any integer between - l and l. Put all of the information. L 2 f.
Principal Quantum Number n2. Now its your turn could you do the electron configuration and get the quantum numbers of the following elements. For every value of n there can be n values of l ranging from 0 1 2 3.
If l 2 m can be -2 -1 0 1 or 2. If l 2 m can be either -2 -1 0 1 or 2. We can establish the relation between m l and n.
Azimuthal quantum number l 2. Magnetic quantum number - m 2.
How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
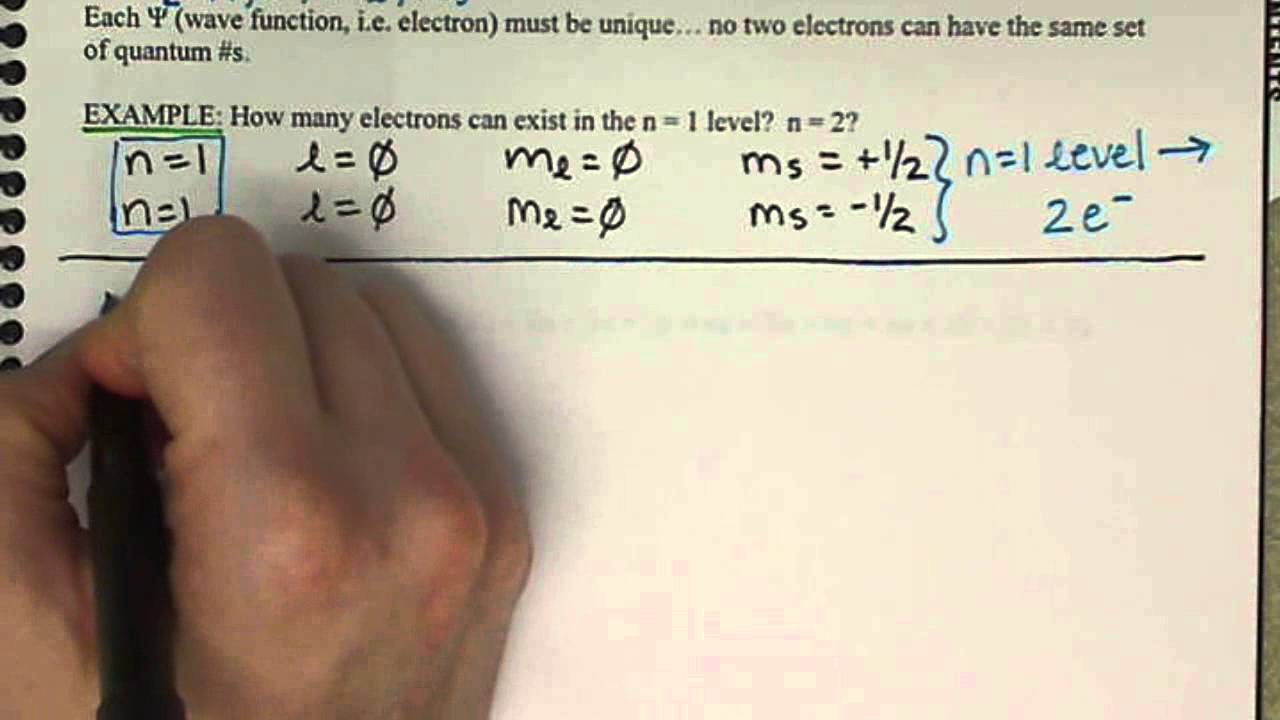
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube
The Magnetic Quantum Number Ml Youtube

Quantum Numbers The Easy Way Youtube
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium