Hence the correct set of quantum numbers is. M s 1 2.

Example Determining If A Set Of 4 Quantum Numbers Is Allowed Or Not Youtube
N3 l1 m1 or 0 or -1 s½ or -½.

How to write set of quantum numbers. Write the complete set of quantum numbers that represent the valence electrons for the following elements. Each set is ordered n ℓ mℓ ms a 2 2 1 12. 1 0.
Thus ms 1 2. Looking at the n and mℓpairing we find that the absolute values are equal. For unpaired electrons convention assigns the value of 1 2 1 2 for the spin quantum number.
Causey shows you step by step how to write quantum numbers for specific electrons in certain elements. G 2 1 1 12. M_s 12 - spin-up electron.
M 0. Finally the spin quantum number m_s describes the spin of the electron. L s the possible values of m l and that does not imply that m l.
For you electron you can select m_s 12. The values of all quantum no. Example 2Identify which sets of quantum numbers are valid for an electron.
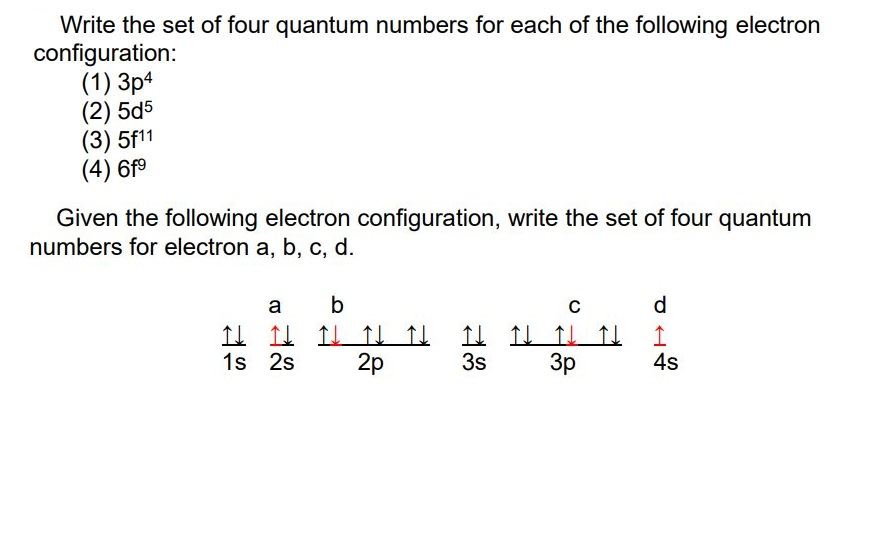
N principle quantum number 2 l angular momentum quantum number 0 ml magnetic quantum number 0 ms spin quantum number 12 or -12. Find Carbon on the Periodic Table. Write the values for the quantum numbers for the bold electron in the following diagrams.
M_s -12 - spin-down electron. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that. N3 - located on the third energy level.
Quantum numbers are a set of 4 imaginary numbers which explain the position and spin of electrons in an atom it can not explain an atom as a whole Iodine has 53 electrons so there are 53 sets of. The spin quantum number signified by the letter s is one of four quantum numbers that characterize the quantum state of an electron including a the. It can have ant positive value 123 and so onThe energy of an electron in an atom depends on nThe smaller the.
Periodic Table for Example Problems. M 0. 1 H - Hydrogen.
45 from Chapter six is asking us to write a set of quantum numbers for each of the electrons with an end of four in the selenium. 2 He - Helium. 1 0.
This video provides 3 example practic. Should be always either 12 or -12. It is an incorrect set of quantum numbers.
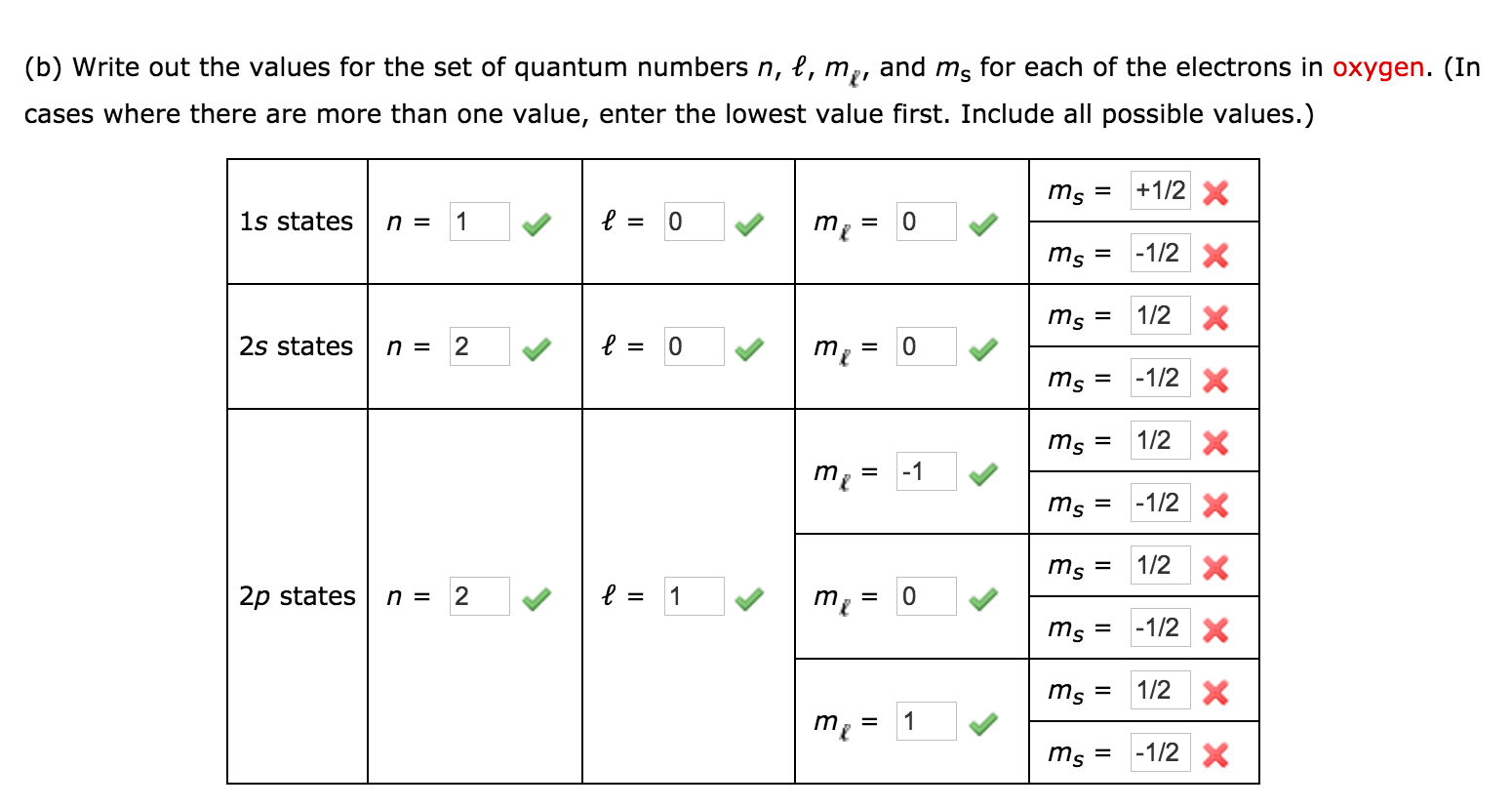
The three p orbitals are degenerate so any of these ml values is correct. Quantum Numbers and Atomic Orbitals By solving the Schrödinger equation Hψ Eψ we obtain a set of mathematical equations called wave functions ψ which describe the probability of finding electrons at certain energy levels within an atom. The correct set of quantum numbers among the given options is.
N 2 ℓ 1 mℓ 2 ms 12. Here you have. 1Principal quantum number nThis quantum number is the one on which the energy of an electron in an atom principally depends.
All you need to know are. See anytime if we see a set of quantum numbers we must see that always. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12.
There are a large number of quantum numbers present for an elementFor Scandium the quantum numbers are n 3 l 2 ml -2 and ms 12. The allowed values and general meaning of each of the four quantum number of an electron numbers of an electron in an atom are as follows. Writing Quantum Numbers.
Therefore n 3 and for a p -type orbital l 1. So the quantum number set that describes this electron will be. 3 1 -1 ½ ie correct option is 3.
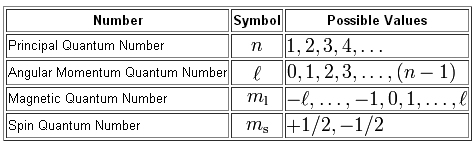
Element Atomic Number Element Symbol Element Name Element Element Quantum Numbers 1. The principal quantum number n the orbital angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms. This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion.
L2 - located in the d-subshell. It has an atomic number of 6. Quantum numbers are also used to understand other characteristics of atoms such as ionization energy and the atomic radius.
The angular momentum quantum number l also referred to as the secondary quantum number or azimuthal quantum number describes the shape of the orbital that an electron occupies. The complete set of quantum numbers that describes this particular electron will be. N5 l0 m_l 0 m_s 12.
This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. Identify the 4 quantum numbers for the last valence electron in a Carbon atom. He n1 l 0 ml 0 ms12.
The ml value could be 1 0 or 1. S 1 2 for first electron n 3. And spin quantum no.
A wave function for an electron in an atom is called anatomic orbital. S 1 2 for second electron. The four sets of quantum numbers principal azimuthal magnetic angular momentum and spin are frequently utilized in order to describe a.
M_l -1 - located in the 3d_xy orbital. So before we start this question it is important to refer to the periodic table to see how many Vaillant selections. The lowest possible value of l is 0 and its highest possible value depending on the principal quantum number.
N 3. M_s -12 - has spin-down since its assumed that the first electron placed in. Thats the selenium Adam has in this case it is six because were looking at selenium.
In atoms there are a total of four quantum numbers.

Quantum Numbers Valid And Invalid Sets Youtube
Quantum Numbers Introduction To Chemistry
Solved Write The Set Of Four Quantum Numbers For Each Of The Chegg Com

Solved Each Electron In An Atom May Be Described By A Set Of Chegg Com
Solved Write Out The Values For The Set Of Quantum Numbers Chegg Com