What are quantum number sets. 213 1 2 is invalid because ml is outside the range of l.
What are 4 quantum No of 19th electron of CU.

What is the set of quantum numbers of li3. 7 In f compare the mℓto the ℓ value. The quantum numbers for electron 1 hydrogen are. Quantum numbers are also used to understand other characteristics of atoms such as ionization energy and the atomic radius.
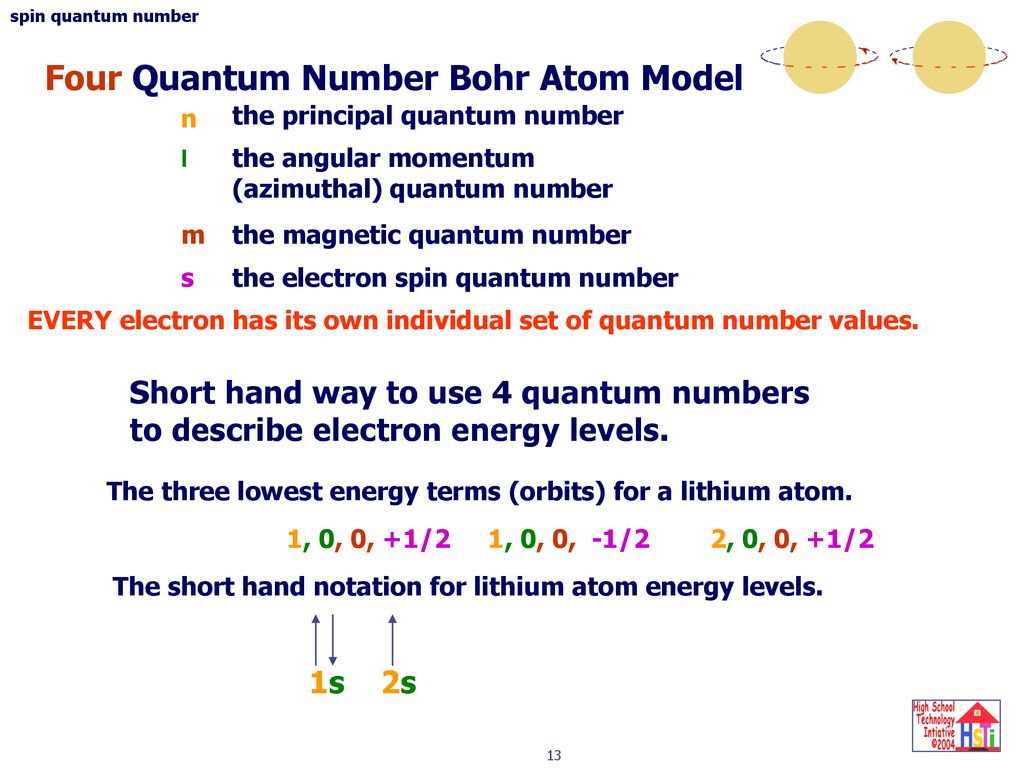
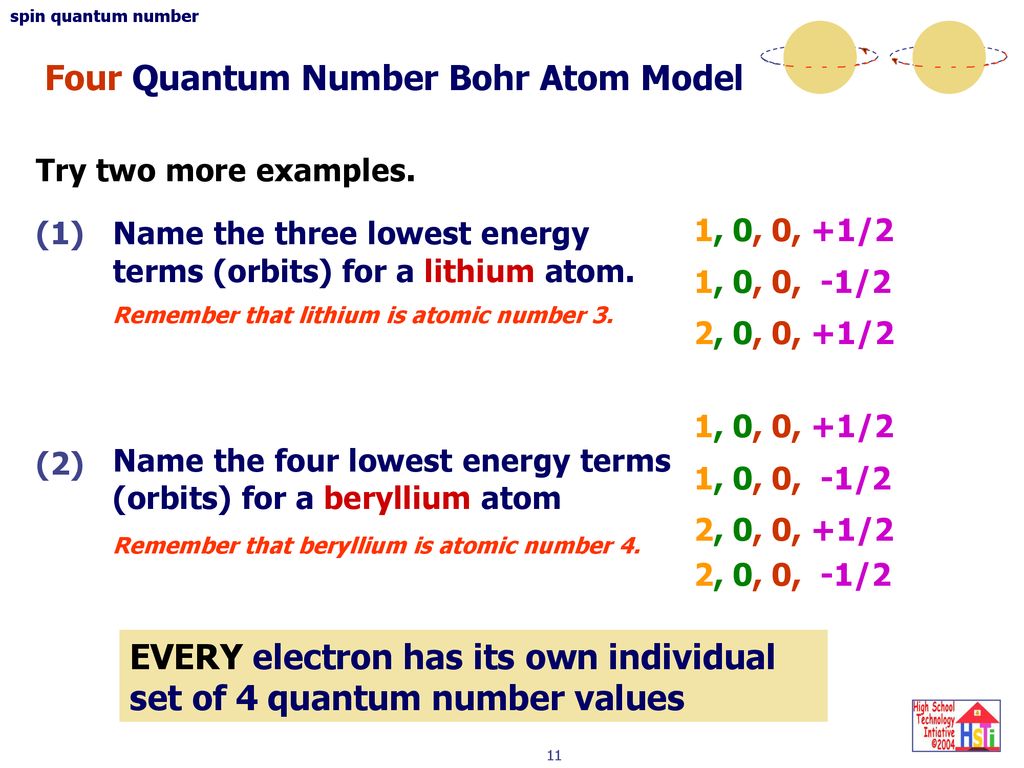
Several rules apply here. A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. Principal quantum number for Lithium is n 12 n 1 2.
N 1 in integer increments. The set of quantum number n 3l 2ml 0. This number divides the subshell into individual orbitals which hold the electrons.
For n 3 l 0 1 2 For l 0 ml 0 For l 1 ml -1 0 or 1 For l 2 ml -2 -1 0 1 or 2 There are 9 ml values and therefore 9 orbitals with n 3. Specifies the orientation in space of an orbital of a given energy n and shape l. The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers.
The quantum numbers provided are n 3 and l 2. 9 O of 1 point earned 3 attempts remaining What is the noble gas electron configuration of Ca. L2 means that the electron is.
3 2 1 0 1 2 3. N 3 l 0 2 b. Since it is given as having a value of 3 this set of quantum numbers is not a valid one.
Si14 n I m ms. The correct set of quantum numbers among the given options is. Quantum numbers are discussed in the final minute but there is info through the entire video youll use to determine quantum numbers.
You have done nothing to indicate a value for m. The magnetic quantum number m can be any integer between -l and l. Thus the s subshell has only one orbital the p subshell has three orbitals and so on.
11 L O of 1 point earned 3 attempts remaining. Or if you need more Quantum Numbers. Which of the following sets of quantum numbers can describe a 4s electron.
Spin Quantum Number practice you can also practice Quantum Numbers. However m is required to be an integer satisfying l m l so m could be any of 1 0 or 1. Identify the set of quantum numbers of each the following elements.
Which is the correct set of 4 quantum numbers. N1 l0 ml0 s-12. The allowed values of nare therefore 1 2 3 4 and so on.
If l 2 m can be either -2 -1 0 1 or 2. Therefore a maximum number of 10 electrons can share these two quantum numbers in an atom. N 3 l 1 6 c.
The set of quantum number. Follow the rules for allowable quantum numbers found in the text. N3 l1 m1 or 0.
Lithium has electron configuration 1s2 2s1Quantum numbers would be. The 19th electron of Cr will reside in 4s orbital. Hence the correct set of quantum numbers is.
The principal quantum number n the orbital angular momentum quantum number l the magnetic quantum number m l and the electron spin quantum number m s. N is the shell number which you indicated to be 3. 10 L O of 1 point earned 3 attempts remaining Which of the following is the electron configuration for Mn.
State the number of possible electrons described by the following quantum numbers a. Ml l l 1 10 1l 1l ms 12. Represents the electron and its spin.
Rules Governing the Allowed Combinations of Quantum Numbers. Second quantum numbers tell us the angular momentum of the electron. Lithium has 3 3 electrons z 3 z 3.
This tells us that the electron is in the third energy level or shell. Represents the number of orbits possible. Li3 n I m ms 2.
In atoms there are a total of four quantum numbers. The mℓvalue of 5 is not permitted in this set of quantum numbers. Lmax n 1.
M l -l 0 l. M l is a range of l. Azimuthal quantum number for Lithium is calculated by l n1 l n 1.
Ca20 n I m ms 5. Ms Spin Quantum number. What type of orbital does an electron with n 3 and L 2 occupy.
There is a lot of info youll need to understand. Our tutors have indicated that to solve this problem you will need to apply the Quantum Numbers. Spin Quantum Number practice problems.
Two possibilities 12 -12 2. - Ill recommend this video to help. Msis allowed to only be values of 12or 12.
Ml can be integers from -l through 0 to l. N4 l0 s m0 s12 upward. 0 1 2 3 and so on.
Each electron in an atom has a unique set of quantum numbers. When ℓ 3 there are seven permissible mℓvalues. Hence the correct set of quantum numbers is.
The three quantum numbers n l and m that describe an orbital are integers. According to the Pauli Exclusion Principle no two electrons can share the same combination of four quantum numbers. First quantum numbers are principle quantum numbers in your case n3.
If n 3 for example l can be either 0 1 or 2. What is the set of correct quantum numbers n ℓ m ℓ for the last electron P. The angular quantum number l can be any integer between 0 and n 1.
There are four quantum numbers namely principal azimuthal magnetic and spin quantum numbers. N 3 l 2 ml -1 2 d. N3 l1 m1 or 0 or -1 s½ or -½.
Principal quantum number n energy level in orbitals and its value could be any positive integer starting from 1 to infinity. The principal quantum number n cannot be zero. This is because no electrons in an atom can have the same set of quantum numbers due to the parameters that quantum numbers set.
The angular quantum number l can be any integer between 0 and n- 1. You can view video lessons to learn Quantum Numbers. N1 l0 ml0 s12.
There are 2l1 orbitals in each subshell. 340 1 2 is invalid because l n. Ml Magnetic quantum number.
What is set of quantum number. Example 5A hydrogen atom has n 5 and mℓ 2. For the format nlmlms.
N 5 l 0 ml -2 ms -12 Not possible. L 0 in integer increments. L values can be integers from 0 to n-1.
The other three sets are valid and can correctly describe an electron. C3 n I m ms 3. The quantum numbers can be given as.
Spin Quantum Number concept. L is the angular momentum quantum number which is 0 for s 1 for p your case 2 for d 3 for f 4 for g. Magnetic Quantum Number m l.
Ne10 n I m ms 4.

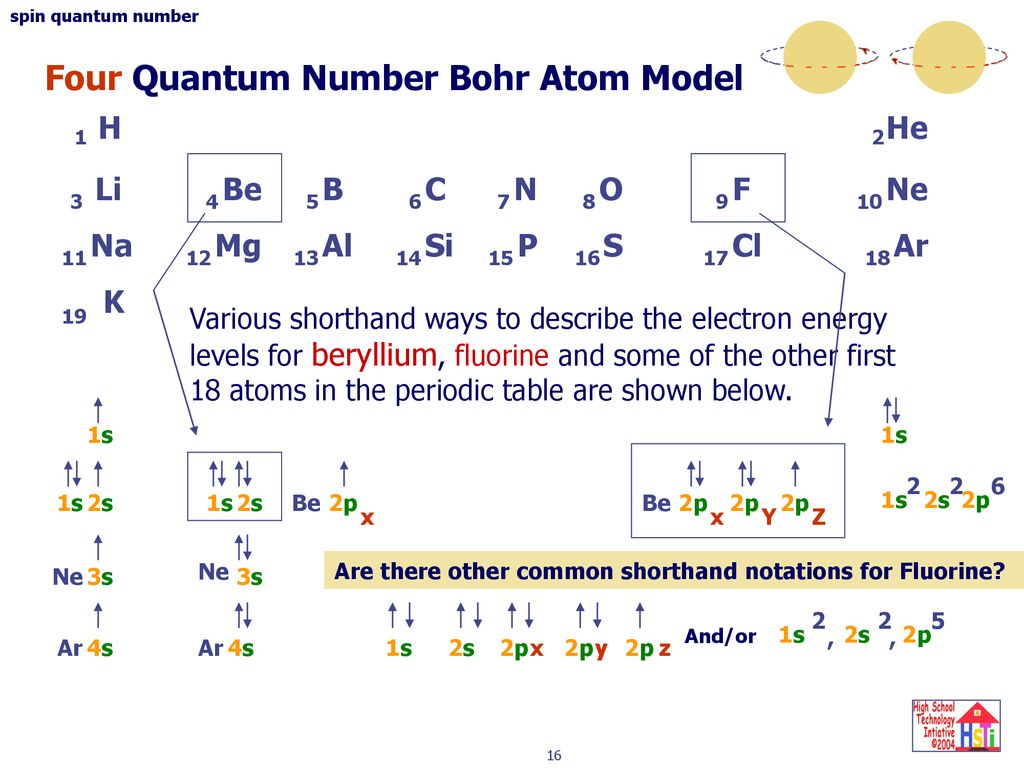
Li Lithium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
Lithium The Periodic Table At Knowledgedoor
The Spin Quantum Number Ppt Download
The Spin Quantum Number Ppt Download
The Spin Quantum Number Ppt Download