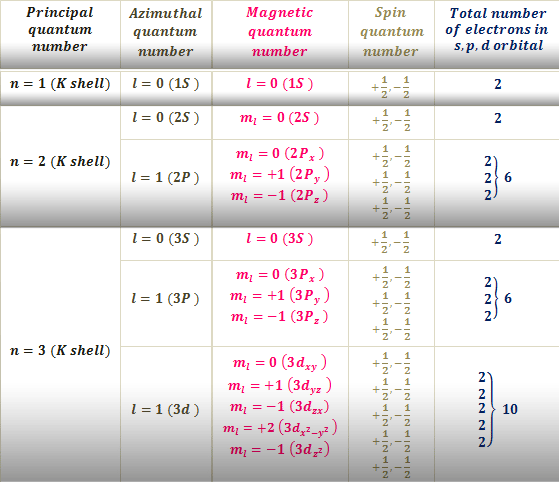
The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same. PRINCIPAL QUANTUM NUMBER n - Represents the main energy level or shell occupied by an electron.
What Will Be The Quantum Numbers For The Last Electron Of Sodium Atom Quora
It relates to principal quantum number and has value zero to n-1 integer.
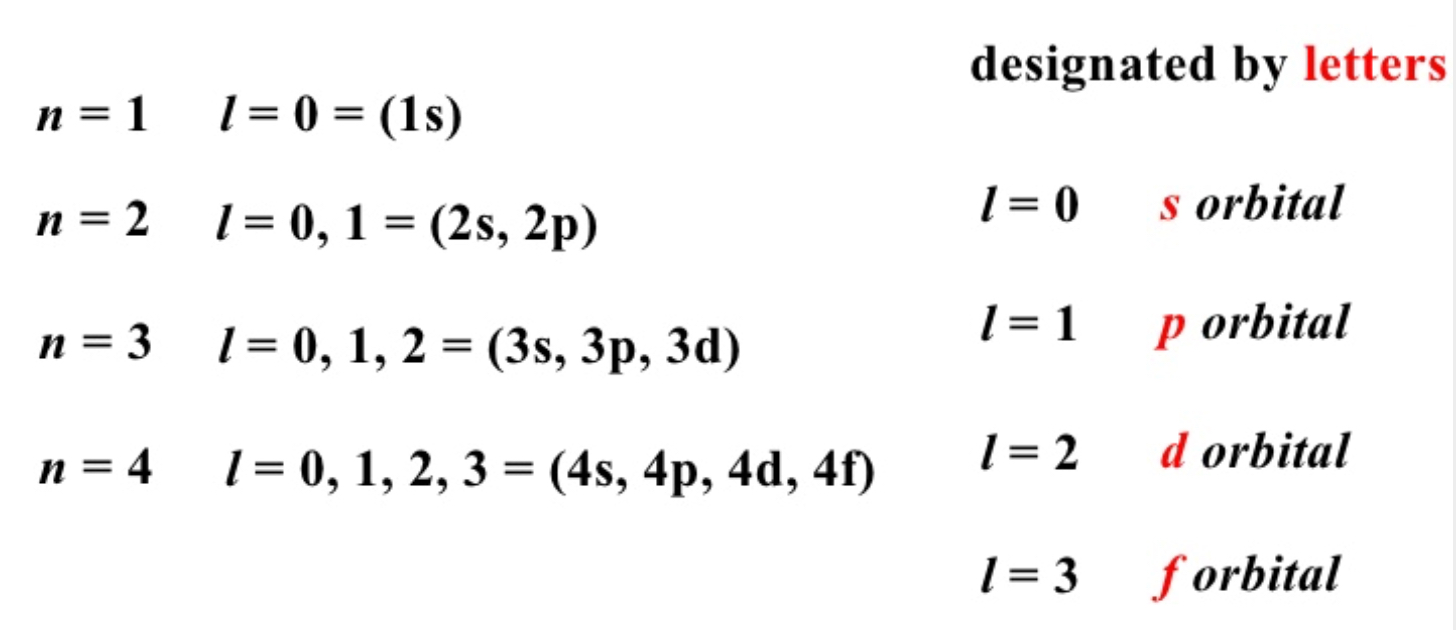
How to find 4 quantum numbers of an element. N ℓ m ℓ and m s. Find Tungsten on the Periodic Table. Layer f - l 3.
Atomic number 24 and copper Cu. To completely describe an electron in an atom four quantum numbers are needed. Principal Quantum Number n.
The correct set of quantum numbers among the given options is. Ions and isotopes of elements are not shown in periodic table. SECONDARY QUANTUM NUMBER l - Represents the energy sublevel or.
Layer p - l 1. The four quantum numbers used to describe the electrons are n2 ℓ1 m1 0 or -1 and s12 the electrons have parallel spins. Find period and group of 24 X.
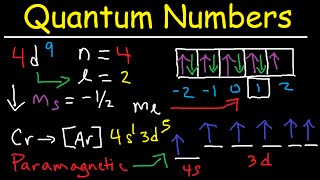
Energy n angular momentum ℓ magnetic moment mℓ and spin ms. The first three n l m l specify the particular orbital of interest and the fourth m s specifies how many electrons can occupy that orbital. For example Calculate the set of quantum numbers for the 19th electron in chromium.
Since the spin can be 12 or 12 there are two combinations. Atomic number 29 among others. Get instant feedback extra help and step-by.
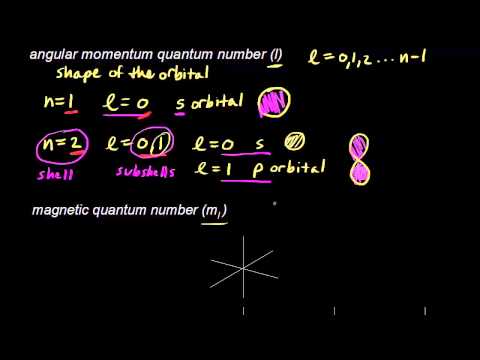
N 1 2 3 8. The shapes of orbital are determined by angular momentum quantum. You will understand how to find the principal quantum number n the angular.
Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that can occupy the orbital. I dont understand how to determine the four different quantum numbers if you are given a certain element from the periodic table like lithium for example. Value is a guess based on periodic table trend.
However we do find exceptions to the order of filling of orbitals that are shown in the previous lessonFor instance the electron configurations shown in the previous lesson of the transition metals chromium Cr. It is always a positive integer that is n 1 2 3. 119 rows This Quantum Numbers chart table gives the Quantum Numbers of all.
The periodic table can be a powerful tool in predicting the electron configuration of an element. The values of four quantum numbers of valence electron of an element are n4 l0 m0 and s12. 3 1 -1 ½ ie correct option is 3.
How to find the four quantum numbers of an electron given an element. Layer s - l 0. Identify the 4 quantum numbers for the last valence electron in a Tungsten atom.
Value is a guess based on periodic table trend. From here on how do I decide what would the principle quantum number be. Groups and Periods of elements are found according to their neutral states.
Log in or register to post comments. Practice Identify the 4 Quantum Numbers for an Elements Last Valence Electron with practice problems and explanations. For example if the electron configuration ends in 4s 2 the principal quantum number will be 4.
This number coincides with the number of the last level of the electron configuration. Value is a guess based on periodic table trend. The element is.
Ill explain the four different types of quantum numbers you will need to know. This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. Periodic Table for Example Problems Step 1.
Likewise what are the four quantum numbers for electrons and how are they defined. How do I find the set of quantum numbers for a specific electron in an element. N 2 l 1 m l 0 m s.
The first quantum number describes the electron shell or energy level of an atom. Value is a guess based on periodic table trend. Layer d - l 2.
L 1 the orbital is s l 2 the orbital is p l 3 the orbital is d l 4 the orbital is f The values of l determine the angular momentum of an electron which has kinetic energy due to angular motion. 24 X1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4. Each electron in an atom is described by four different quantum numbers.
Period and 426 B group. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12. This video provides 3 example practic.
There are four quantum numbers. This number depends on the last layer that has been filled. The electronic configuration of the 19th electron is 1s2 2s2 2p6 3s2 3p6 4s1.
If X 2 ion has 10 electrons find its group and period number.
Quantum Numbers Video Quantum Physics Khan Academy

Quantum Numbers The Easy Way Youtube
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium
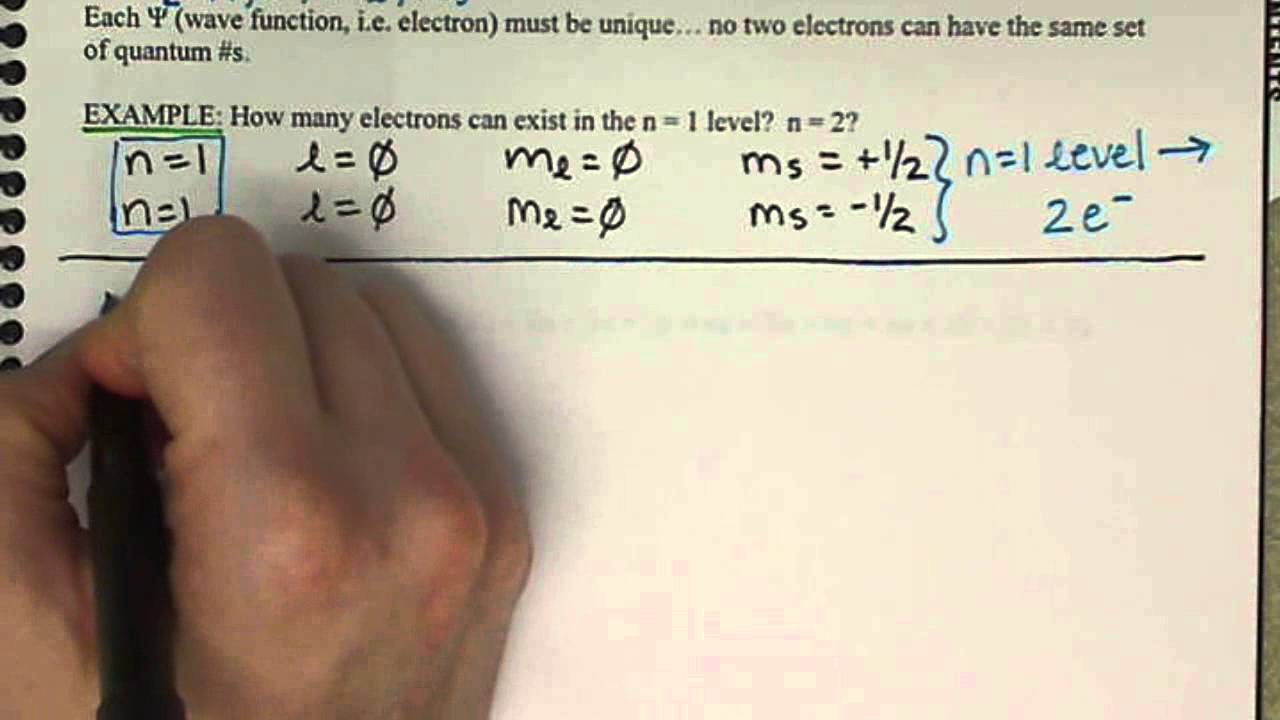
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 Youtube
Quantum Numbers The Easy Way Youtube