N 3 l 2 ml -1 2 d. An electron can spin in only one of two directions sometimes called up and down.
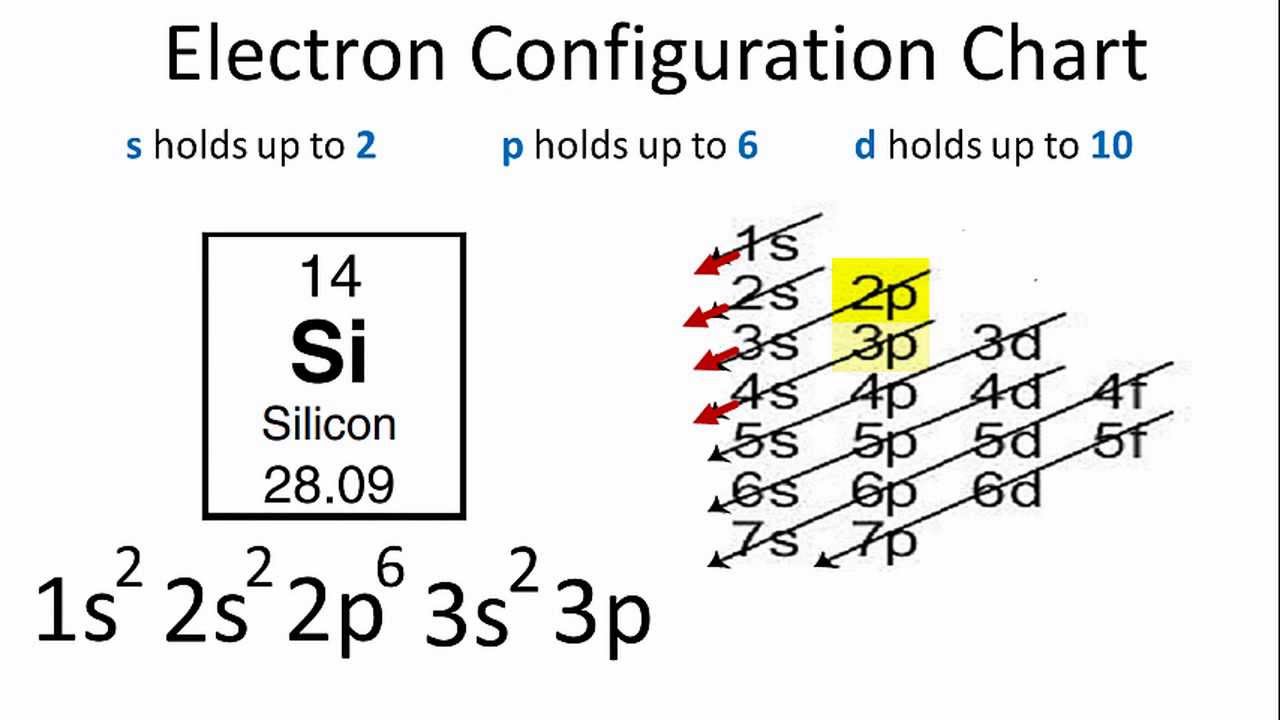
Electron Configuration For Silicon Si
15 P - Phosphorus.

What is the set of quantum numbers for silicon si. This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion. So pincipal quantum number is 3. So in silicon how many electrons will have n 3 as one of their quantum numbers.
The third shell of Si has 4 electrons - 2 in the s orbital and 2 in p orbitals. How to Write the Electron Configuration for Silicon Si In order to write the Silicon electron configuration we first need to know the number of electrons for the Si atom there are 14 electrons. The answer is 4.
The Pauli exclusion principle Wolfgang Pauli Nobel Prize 1945 states thatno two. Silicons valence electrons are in shell 3. N 5.
452 would be 0300-12 240012 411 12 00 12. There are two 3 s electrons and two 3 p electrons. The answer is 4.
The electron affinity of fluorine is essentially equal to. These valence electrons are responsible for all chemical bonds between atoms. Ml Magnetic quantum number.
Si C Al D Se E P Ans. Likewise what are the quantum numbers for silicon. This means that a neutral silicon atom has 14 protons inside its nucleus and 14 electrons surrounding its nucleus.
Such a relationship is called A amphoterism. See full answer below. Si Silicon 280855 15 P Phosphorus 30973761 16 S Sulfur 32066 17 Cl Chlorine 354527 18 Ar Argon 39948 19 K Potassium 390983 20 Ca Calcium 40078 21 Sc Scandium 44955910 22 Ti Titanium.
Now notice that silicon has an atomic number equal to 14. Represents the electron and its spin. Principal quantum number n azimutal quantum number magnetic quantum number m spin quantum number sd.
So in silicon how many electrons will have n 3 as one of their quantum numbers. N 3 l 0 2 b. The s-electrons have orbital quantum number 0 the p-electrons 1.
For silicon the valence shell corresponds to. 100½ and -½ 200 ½ and -½ 211 ½ and -½ 210 ½ and -½ 21-1 ½ and -½ 3 0 0½ and -½ 311½ 310½ 2. Each electron in an atom has a unique set of four quantum numbers.
What is the 14th electron of silicon. Question 3 The quantum numbers that would go with the element Silicon Si would be O 310 12 O 300 -12 O 3 1-1 12 O 3 10 -12 Question 4 the quantum numbers for the element that has its last electron as. What is silicons flammability in numbers.
M s ½ or -½. Spin quantum number ms can only have two possible values ½ spin up electron or ½ spin down electrons Note that 1 orbital can hold a maximum of 2 electrons 1 spin up ½ and 1 spin down ½ For the 2s electrons. For silicon the valence shell corresponds to a principle quantum number n of 3.
All start with 3 so all will have a principal quantum number of 3. D subshell 5 orbitals. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one.
Eq1 n 3 l 0 m_l. 16 S - Sulfur. The answer is 4.
F subshell 7 orbitals. Ms Spin Quantum number. Represents the number of orbits possible.
There are two 3s electrons and two 3p electrons. 17 Cl - Chlorine. Two possibilities 12 -12 2.
Write the 14 sets of quantum numbers that describe the 14 electrons of silicon Si. State the number of possible electrons described by the following quantum numbers a. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12.
When we write the configuration well put all 14 electrons in orbitals around the nucleus of the Silicon atom. The outermost energy level that in the ground state of the atom contains electrons is called the valence shell of the atom. QUANTUM NUMBERS WORKSHEET KEY 1.
N 3 l 1 6 c. No two electrons can have the same four quantum numbers. Spin Quantum Number m s.
In your case you need to find the set of quantum numbers that describe the 13th electron in a neutral silicon atom. S subshell ℓ 0. M l is a range of l.
Specifies the orientation of the spin axis of an electron. D the periodic law. A possible set of quantum numbers to describe an electron in a 3d subshell is A n 3 l 0 m l 0 m s.
The s-electron magnetic quantum numbers are 0 spin. Technical data for Silicon Click any property name to see plots of that property for all the elements. Their quantum numbers look like this.
14 Si - Silicon. Its electron configuration is 3s2 3p2. Therefore you can say that the two electrons added last to the electron configuration of a neutral silicon atom will have the following sets of quantum numbers n 3 l1 m_l -1 m_s 12 This electrons is located on the third energy level in the 3p subshell in the 3p_x orbital and has spin-up.
4s n 4.

B 3 Electron Configuration Boron Ion Electron Configuration Electrons Chemistry

Nitrogen Oxides Name And Structure Chemistry Notes Chemistry Classroom Chemistry

Electron Configurations The Four Quantum Numbers Video Lesson Transcript Study Com

118 Element Color Periodic Table Stars And Nebula By Sciencenotes Periodic Table Science Notes Element Chart
